Biochimie ( IF 3.3 ) Pub Date : 2020-07-24 , DOI: 10.1016/j.biochi.2020.07.008 Gabriela Gontijo Montandon 1 , Juliana Silva Cassoli 2 , Steve Peigneur 3 , Thiago Verano-Braga 4 , Daniel Moreira Dos Santos 1 , Ana Luiza Bittencourt Paiva 5 , Éder Ricardo de Moraes 6 , Christopher Kushmerick 6 , Márcia Helena Borges 5 , Michael Richardson 5 , Adriano Monteiro de Castro Pimenta 1 , Frank Kjeldsen 7 , Marcelo Ribeiro Vasconcelos Diniz 5 , Jan Tytgat 3 , Maria Elena de Lima 8
|
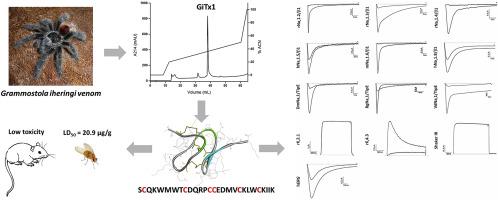
Spider venoms, despite their toxicity, represent rich sources of pharmacologically active compounds with biotechnological potential. However, in view of the large diversity of the spider species, the full potential of their venom molecules is still far from being known. In this work, we report the purification and structural and functional characterization of GiTx1 (β/κ-TRTX-Gi1a), the first toxin purified from the venom of the Brazilian tarantula spider Grammostola iheringi. GiTx1 was purified by chromatography, completely sequenced through automated Edman degradation and tandem mass spectrometry and its structure was predicted by molecular modeling. GiTx1 has a MW of 3.585 Da, with the following amino acid sequence: SCQKWMWTCDQKRPCCEDMVCKLWCKIIK. Pharmacological activity of GiTx1 was characterized by electrophysiology using whole-cell patch clamp on dorsal root ganglia neurons (DRG) and two-electrode voltage-clamp on voltage-gated sodium and potassium channels subtypes expressed in Xenopus laevis oocytes. GiTx1, at 2 μM, caused a partial block of inward (∼40%) and outward (∼20%) currents in DRG cells, blocked rNav1.2, rNav1.4 and mNav1.6 and had a significant effect on VdNav, an arachnid sodium channel isoform. IC50 values of 156.39 ± 14.90 nM for Nav1.6 and 124.05 ± 12.99 nM for VdNav, were obtained. In addition, this toxin was active on rKv4.3 and hERG potassium channels, but not Shaker IR or rKv2.1 potassium channels. In summary, GiTx1 is a promiscuous toxin with multiple effects on different types of ion channels.
中文翻译:

GiTx1(β/κ-theraphotoxin-Gi1a),一种从巴西狼蛛Grammostola iheringi(Mygalomorphae,Theraphosidae)的毒液中提取的新型毒素:电压门控离子通道的分离,结构评估和活性。
蜘蛛毒液尽管具有毒性,却代表了具有生物技术潜力的药理活性化合物的丰富来源。然而,鉴于蜘蛛种类的多样性,其毒液分子的全部潜力仍是未知的。在这项工作中,我们报告了GiTx1(β/κ-TRTX-Gi1a)的纯化,结构和功能表征,GiTx1是从巴西狼蛛蜘蛛Grammostola iheringi的毒液中纯化得到的第一种毒素。。GiTx1通过色谱纯化,通过自动Edman降解和串联质谱法进行完全测序,并通过分子建模预测其结构。GiTx1的MW为3.585 Da,具有以下氨基酸序列:SCQKWMWTCDQKRPCCEDMVCKLWCKIIK。GiTx1的药理活性由电生理学表征,使用全细胞膜片钳夹在背根神经节神经元(DRG)上,并用两电极电压钳夹在非洲爪蟾卵母细胞中表达的电压门控钠和钾通道亚型上。GiTx1的浓度为2μM,在DRG细胞中引起部分内向电流(〜40%)和外向电流(〜20%),阻断rNav1.2,rNav1.4和mNav1.6,并对VdNav产生显着影响,蛛网膜钠通道亚型。IC 50对于Nav1.6获得了156.39±14.90 nM的值,对于VdNav获得了124.05±12.99 nM的值。此外,该毒素对rKv4.3和hERG钾通道具有活性,但对Shaker IR或rKv2.1钾通道不具有活性。总之,GiTx1是一种混杂毒素,对不同类型的离子通道具有多种作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号