当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Balancing the transesterification reactivity of isosorbide with diphenyl carbonate: preferential activation of exo-OH
Polymer Chemistry ( IF 4.1 ) Pub Date : 2020-07-23 , DOI: 10.1039/d0py00764a Ming Zhang 1, 2, 3, 4, 5 , Yifei Tu 1, 2, 3, 4, 5 , Zibo Zhou 1, 2, 3, 4, 5 , Guozhang Wu 1, 2, 3, 4, 5
Polymer Chemistry ( IF 4.1 ) Pub Date : 2020-07-23 , DOI: 10.1039/d0py00764a Ming Zhang 1, 2, 3, 4, 5 , Yifei Tu 1, 2, 3, 4, 5 , Zibo Zhou 1, 2, 3, 4, 5 , Guozhang Wu 1, 2, 3, 4, 5
Affiliation
|
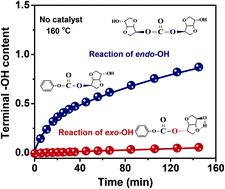
The exo-hydroxyl group (exo-OH) on isosorbide (ISB) has long been asserted as a highly reactive moiety compared with the endo-hydroxyl group (endo-OH). In this study, calculations based on density functional theory and experiments without adding catalysts reveal that endo-OH has strong nucleophilic ability, and in the case of transesterification with diphenyl carbonate, the nucleophilic attack surmounts steric hindrance in rendering endo-OH more reactive than exo-OH. The kinetics of transesterification with different catalysts is investigated to determine the catalytic reactivity and molecular structure evolution. The results show that preferential activation of exo-OH moieties can be achieved either by reducing the coordination ability of catalytic cations (lg β1) or by enhancing the alkalinity of catalytic anions. Despite the low reactivity of exo-OH on ISB monomers, terminal exo-OH on carbonate oligomers exhibits higher reactivity than terminal endo-OH, justifying the experimental fact that ISB-based polycarbonates (ISB-PCs) are mostly terminated by endo-OH. Balancing the reactivity between endo-OH and exo-OH can be promoted by increasing the reaction temperature, thus accelerating transesterification to a high equilibrium constant. These findings help to clarify the mechanism of ISB transesterification and provide new strategies for the synthesis of high-molecular-weight ISB-PCs.
中文翻译:

平衡异山梨醇与碳酸二苯酯的酯交换反应性:exo-OH的优先活化
长期以来,异山梨醇(ISB)上的exo-羟基(exo -OH)一直被认为是与内羟基(endo -OH)相比具有高反应性的部分。在这项研究中,根据密度泛函理论和不添加催化剂的实验进行的计算表明,内-OH具有很强的亲核能力,在与碳酸二苯酯进行酯交换反应的情况下,亲核的攻击要克服位阻,从而使内-OH的反应性比外源性的要高。-哦。研究了用不同催化剂进行酯交换反应的动力学,以确定催化反应性和分子结构的演变。结果表明的那优先激活外可以通过降低催化阳离子的配位能力(LG任一实现-OH结构部分 β 1)或通过增强催化阴离子的碱度。尽管低反应性外切于ISB单体,末端-OH外-OH上碳酸酯低聚物的反应性显示出高于终端内切-OH,证明实验事实,即基于ISB的聚碳酸酯(ISB-PC)的主要是由封端的内切-OH。平衡的反应性内切-OH和外-OH可通过提高反应温度来促进,从而将酯交换反应加速至高平衡常数。这些发现有助于阐明ISB酯交换的机理,并为合成高分子量ISB-PCs提供了新的策略。
更新日期:2020-09-01
中文翻译:

平衡异山梨醇与碳酸二苯酯的酯交换反应性:exo-OH的优先活化
长期以来,异山梨醇(ISB)上的exo-羟基(exo -OH)一直被认为是与内羟基(endo -OH)相比具有高反应性的部分。在这项研究中,根据密度泛函理论和不添加催化剂的实验进行的计算表明,内-OH具有很强的亲核能力,在与碳酸二苯酯进行酯交换反应的情况下,亲核的攻击要克服位阻,从而使内-OH的反应性比外源性的要高。-哦。研究了用不同催化剂进行酯交换反应的动力学,以确定催化反应性和分子结构的演变。结果表明的那优先激活外可以通过降低催化阳离子的配位能力(LG任一实现-OH结构部分 β 1)或通过增强催化阴离子的碱度。尽管低反应性外切于ISB单体,末端-OH外-OH上碳酸酯低聚物的反应性显示出高于终端内切-OH,证明实验事实,即基于ISB的聚碳酸酯(ISB-PC)的主要是由封端的内切-OH。平衡的反应性内切-OH和外-OH可通过提高反应温度来促进,从而将酯交换反应加速至高平衡常数。这些发现有助于阐明ISB酯交换的机理,并为合成高分子量ISB-PCs提供了新的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号