当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, optical and electrochemical properties of propeller-type 3,5,8-trithienyl-BODIPY dyes
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2020-07-23 , DOI: 10.1039/d0qm00494d Shuhei Tsumura 1, 2, 3, 4, 5 , Kazuki Ohira 1, 2, 3, 4, 5 , Kosuke Hashimoto 1, 2, 3, 4, 5 , Keiichi Imato 1, 2, 3, 4, 5 , Yousuke Ooyama 1, 2, 3, 4, 5
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2020-07-23 , DOI: 10.1039/d0qm00494d Shuhei Tsumura 1, 2, 3, 4, 5 , Kazuki Ohira 1, 2, 3, 4, 5 , Kosuke Hashimoto 1, 2, 3, 4, 5 , Keiichi Imato 1, 2, 3, 4, 5 , Yousuke Ooyama 1, 2, 3, 4, 5
Affiliation
|
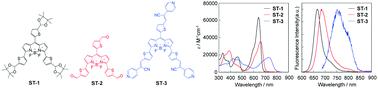
We designed and developed propeller-type 3,5,8-trithienyl-BODIPY dyes ST-1, ST-2, and ST-3, which have the same three units of tetramethyl-2-(thiophen-2-yl)-1,3-dioxolane, thiophene-2-carbaldehyde, or 2-(pyridin-4-yl)-3-(thiophen-2-yl)acrylonitrile at the 3-, 5-, and 8-positions on the BODIPY skeleton, respectively. In order to get an insight into the impacts of 3,5,8-trithienyl substituents on the BODIPY core on the optical and electrochemical properties, we performed the photoabsorption and fluorescence spectroscopy, Lippert–Mataga plots, cyclic voltammetry (CV) and density functional theory (DFT) calculations for ST-1, ST-2, and ST-3. The photoabsorption and fluorescence maxima (λabsmax and λflmax) of the three 3,5,8-trithienyl-DODIPY dyes exhibit bathochromic shifts in the order of ST-1 (ca. 640 and 660 nm) < ST-2 (ca. 660 and 680 nm) < ST-3 (ca. 730 and 750 nm), which appear in significantly longer wavelength regions compared to those of the 3,5,8-triphenyl BODIPY dye. This fact indicates that the expansion of the π-conjugated system by the introduction of thiophene units as spacers at the 3-, 5-, and 8-positions onto the BODIPY core can lead to bathochromic shifts of photoabsorption and fluorescence bands to the red/NIR region. It was found that the photoabsorption and fluorescence bands of the propeller-type 3,5,8-trithienyl-BODIPY dyes are nearly independent of solvent polarity, that is, ST-1, ST-2, and ST-3 show feeble solvatochromic properties. Moreover, we revealed that ST-3 possesses the ability to generate singlet oxygen (1O2) under visible light irradiation, and thus, this result provides useful knowledge in molecular design of efficient BODIPY-based photosensitizers for photodynamic therapy (PDT).
中文翻译:

螺旋桨型3,5,8-三噻吩基-BODIPY染料的合成,光学和电化学性质
我们设计并开发了螺旋桨型3,5,8-三噻吩基-BODIPY染料ST-1,ST-2和ST-3,它们具有相同的三个单元,即四甲基-2-(噻吩-2-基)-1 ,分别在BODIPY骨架的3-,5-和8位上形成一个,3-二氧戊环,噻吩-2-甲醛或2-(吡啶-4-基)-3-(噻吩-2-基)丙烯腈。为了深入了解BODIPY核上3,5,8-三噻吩基取代基对光学和电化学性质的影响,我们进行了光吸收和荧光光谱分析,Lippert-Mataga图,循环伏安法(CV)和密度泛函ST-1,ST-2和ST-3的理论(DFT)计算。光吸收和荧光最大值(λ腹肌最大和λ FL最大值)的三个3,5,8-trithienyl-DODIPY染料的顺序表现出红移ST-1 (约640和660纳米)< ST-2 (约660和680纳米的)< ST-3(约730和750 nm),与3,5,8-三苯基BODIPY染料相比,出现在更长的波长区域。这一事实表明,通过在BODIPY核心的3、5和8位上引入噻吩单元作为间隔基,π共轭体系的扩展会导致光吸收和荧光带向红/红移的红移。近红外区域。发现螺旋桨型3,5,8-三噻吩基-BODIPY染料的光吸收和荧光带几乎与溶剂极性无关,即ST-1,ST-2和ST-3显示弱的溶剂化性质。 。此外,我们发现ST-3具有产生单线态氧(1 O 2),因此,该结果为高效的基于BODIPY的光动力疗法(PDT)光敏剂的分子设计提供了有用的知识。
更新日期:2020-08-27
中文翻译:

螺旋桨型3,5,8-三噻吩基-BODIPY染料的合成,光学和电化学性质
我们设计并开发了螺旋桨型3,5,8-三噻吩基-BODIPY染料ST-1,ST-2和ST-3,它们具有相同的三个单元,即四甲基-2-(噻吩-2-基)-1 ,分别在BODIPY骨架的3-,5-和8位上形成一个,3-二氧戊环,噻吩-2-甲醛或2-(吡啶-4-基)-3-(噻吩-2-基)丙烯腈。为了深入了解BODIPY核上3,5,8-三噻吩基取代基对光学和电化学性质的影响,我们进行了光吸收和荧光光谱分析,Lippert-Mataga图,循环伏安法(CV)和密度泛函ST-1,ST-2和ST-3的理论(DFT)计算。光吸收和荧光最大值(λ腹肌最大和λ FL最大值)的三个3,5,8-trithienyl-DODIPY染料的顺序表现出红移ST-1 (约640和660纳米)< ST-2 (约660和680纳米的)< ST-3(约730和750 nm),与3,5,8-三苯基BODIPY染料相比,出现在更长的波长区域。这一事实表明,通过在BODIPY核心的3、5和8位上引入噻吩单元作为间隔基,π共轭体系的扩展会导致光吸收和荧光带向红/红移的红移。近红外区域。发现螺旋桨型3,5,8-三噻吩基-BODIPY染料的光吸收和荧光带几乎与溶剂极性无关,即ST-1,ST-2和ST-3显示弱的溶剂化性质。 。此外,我们发现ST-3具有产生单线态氧(1 O 2),因此,该结果为高效的基于BODIPY的光动力疗法(PDT)光敏剂的分子设计提供了有用的知识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号