Neuroscience Letters ( IF 2.5 ) Pub Date : 2020-07-23 , DOI: 10.1016/j.neulet.2020.135272 Riccardo Sirtori 1 , Chiara Riva 1 , Carlo Ferrarese 2 , Gessica Sala 1
|
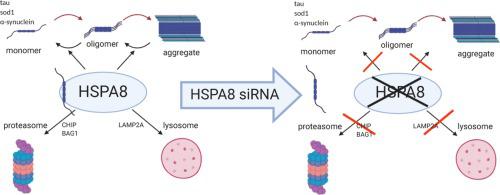
Heat shock protein 70 family was demonstrated to play a critical role in protein homeostasis, a process profoundly impaired in neurodegenerative disorders. Neurodegenerative diseases are characterized by the accumulation of different kind of proteins and the formation of insoluble aggregates which are toxic for neurons. To explore the role of heat shock protein family 70 (in particular HSPA8 and HSPA1A) in the accumulation of proteins implied in neurodegeneration pathogenesis, in this study we verified in human SH-SY5Y neuroblastoma cells how HSPA8 or HSPA1A knock-down can affect protein levels of tau, superoxide dismutase 1 and α-synuclein. We found HSPA8 and HSPA1A reduction caused an increase of tau, superoxide dismutase 1 and α-synuclein protein levels. We also noticed HSPA8 knock-down increased α-synuclein oligomeric forms and mRNA expression. Our results suggest HSPA8 can play an important role in the homeostasis of tau, superoxide dismutase 1 and α-synuclein and in the balance between α-synuclein oligomeric and monomeric forms.
中文翻译:

HSPA8敲低诱导神经退行性疾病相关蛋白的积累。
事实证明,热休克蛋白70家族在蛋白质稳态中起着至关重要的作用,这一过程在神经退行性疾病中大大受损。神经退行性疾病的特征是各种蛋白质的积累和对神经元有毒的不溶性聚集物的形成。为了探讨热休克蛋白家族70(特别是HSPA8和HSPA1A)在神经退行性发病机理中隐含的蛋白质积累中的作用,在本研究中,我们在人SH-SY5Y神经母细胞瘤细胞中验证了HSPA8或HSPA1A敲低如何影响蛋白质水平tau,超氧化物歧化酶1和α-突触核蛋白。我们发现HSPA8和HSPA1A的减少导致tau,超氧化物歧化酶1和α-突触核蛋白蛋白水平增加。我们还注意到HSPA8敲低增加了α-突触核蛋白的寡聚形式和mRNA表达。











































 京公网安备 11010802027423号
京公网安备 11010802027423号