Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-07-23 , DOI: 10.1016/j.jcis.2020.07.097 Buddha R Shrestha 1 , Benoit Liberelle 2 , Frederic Murschel 2 , Enrico O Purisima 3 , Traian Sulea 3 , Gregory De Crescenzo 2 , Xavier Banquy 1
|
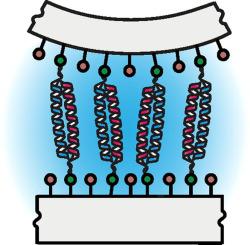
We used the Surface Forces Apparatus to elucidate the interaction mechanism between grafted 5 heptad-long peptides engineered to spontaneously form a heterodimeric coiled-coil complex. The results demonstrated that when intimate contact between peptides is reached, binding occurs first via weakly interacting but more mobile distal heptads, suggesting an induced-fit association process. Precise control of the distance between peptide-coated surfaces allowed to quantitatively monitor the evolution of their biding energy. The binding energy of the coiled-coil complex increased in a stepwise fashion rather than monotonically with the overlapping distance, each step corresponding to the interaction between a quantized number of heptads. Surface forces data were corroborated to surface plasmon resonance measurements and molecular dynamics simulations and allowed the calculation of the energetic contribution of each heptad within the coiled-coil complex.
中文翻译:

从表面力测量中阐明了从头开始的卷曲螺旋复合物的结合机理。
我们使用表面力仪器阐明了被工程改造以自发形成异二聚体卷曲螺旋复合物的5个接枝的5个七肽长肽之间的相互作用机理。结果表明,当肽之间达到紧密接触时,结合首先通过弱相互作用但更易移动的远端七肽发生,这表明诱导装配关联过程。精确控制肽涂层表面之间的距离可以定量监测其结合能量的演变。卷曲螺旋复合物的结合能以逐步的方式而不是随着重叠距离单调增加,每一步对应于定量的七肽之间的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号