当前位置:
X-MOL 学术
›
Chem. Geol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparison of the reaction kinetics and mechanisms of Sb(III) oxidation by reactive oxygen species from pristine and surface-oxidized pyrite
Chemical Geology ( IF 3.6 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.chemgeo.2020.119790 Wenting Wang , Chengjun Zhang , Jun Shan , Mengchang He
Chemical Geology ( IF 3.6 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.chemgeo.2020.119790 Wenting Wang , Chengjun Zhang , Jun Shan , Mengchang He
|
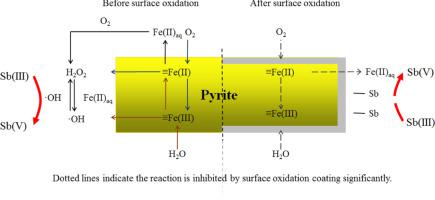
Abstract With the rapid increase in the antimony (Sb) concentration in the environment, the environmental risk and pollution control of Sb has gradually attracted wide attention. The oxidation of Sb by pyrite influences its toxicity and mobility. Pyrite is oxidized by dissolved oxygen (DO) in an aerobic environment to form a surface oxide coating. However, the effect of the surface oxide coating on the reaction activity of pyrite is poorly understood. In this study, the influence of oxidation products on the surface morphology of pyrite and its interfacial reaction with Sb were investigated comprehensively. The amount of surface oxide coating increased with increasing pH. The main oxidation products under acidic conditions were mainly iron (II) hydroxy complex, and those under neutral conditions were iron (III) hydroxy complex. In a suspension of surface-oxidized pyrite (SOP), the oxidation efficiency of Sb(III) decreased significantly, and inhibition became more significant with increasing pH. As a result of the formation of the surface oxide coating, the contents of hydrogen peroxide (H2O2) and hydroxyl radicals (·OH), which are the main oxidants of Sb(III), were found to be lower in the SOP suspension than in pristine pyrite. The reasons are as follows. On the one hand, surface oxidation products decompose H2O2. On the other hand, they retard the production of H2O2 and ·OH by inhibiting Fe(II) release into the solution and O2 diffusion into the surface of pyrite. In addition, the adsorption capacity of Sb was enhanced after aqueous oxidation because Fe hydroxy complex have a strong affinity to Sb and large specific surface area. Aqueous oxidation of pyrite greatly influences the geochemical cycling and existence of Sb in the environment. The study lays a solid foundation for assessing the environmental risk of and developing pollution controls for Sb.
中文翻译:

来自原始和表面氧化黄铁矿的活性氧物种氧化 Sb(III) 的反应动力学和机制的比较
摘要 随着环境中锑(Sb)浓度的迅速升高,Sb的环境风险和污染控制逐渐引起人们的广泛关注。黄铁矿对 Sb 的氧化会影响其毒性和迁移率。黄铁矿在有氧环境中被溶解氧 (DO) 氧化,形成表面氧化物涂层。然而,表面氧化物涂层对黄铁矿反应活性的影响知之甚少。本研究综合研究了氧化产物对黄铁矿表面形貌的影响及其与Sb的界面反应。表面氧化物涂层的量随着pH值的增加而增加。酸性条件下氧化产物主要为羟基铁(II)络合物,中性条件下氧化产物主要为羟基铁(III)络合物。在表面氧化黄铁矿 (SOP) 的悬浮液中,Sb(III) 的氧化效率显着降低,并且随着 pH 值的增加,抑制作用变得更加显着。由于表面氧化物涂层的形成,发现作为 Sb(III) 主要氧化剂的过氧化氢 (H2O2) 和羟基自由基 (·OH) 在 SOP 悬浮液中的含量低于在 SOP 悬浮液中的含量。原始黄铁矿。原因如下。一方面,表面氧化产物分解 H2O2。另一方面,它们通过抑制 Fe(II) 释放到溶液中和 O2 扩散到黄铁矿表面来延缓 H2O2 和·OH 的产生。此外,由于Fe羟基络合物对Sb的亲和力强,比表面积大,水氧化后对Sb的吸附能力增强。黄铁矿的水氧化对环境中Sb的地球化学循环和存在有很大影响。该研究为评估锑的环境风险和制定污染控制措施奠定了坚实的基础。
更新日期:2020-10-01
中文翻译:

来自原始和表面氧化黄铁矿的活性氧物种氧化 Sb(III) 的反应动力学和机制的比较
摘要 随着环境中锑(Sb)浓度的迅速升高,Sb的环境风险和污染控制逐渐引起人们的广泛关注。黄铁矿对 Sb 的氧化会影响其毒性和迁移率。黄铁矿在有氧环境中被溶解氧 (DO) 氧化,形成表面氧化物涂层。然而,表面氧化物涂层对黄铁矿反应活性的影响知之甚少。本研究综合研究了氧化产物对黄铁矿表面形貌的影响及其与Sb的界面反应。表面氧化物涂层的量随着pH值的增加而增加。酸性条件下氧化产物主要为羟基铁(II)络合物,中性条件下氧化产物主要为羟基铁(III)络合物。在表面氧化黄铁矿 (SOP) 的悬浮液中,Sb(III) 的氧化效率显着降低,并且随着 pH 值的增加,抑制作用变得更加显着。由于表面氧化物涂层的形成,发现作为 Sb(III) 主要氧化剂的过氧化氢 (H2O2) 和羟基自由基 (·OH) 在 SOP 悬浮液中的含量低于在 SOP 悬浮液中的含量。原始黄铁矿。原因如下。一方面,表面氧化产物分解 H2O2。另一方面,它们通过抑制 Fe(II) 释放到溶液中和 O2 扩散到黄铁矿表面来延缓 H2O2 和·OH 的产生。此外,由于Fe羟基络合物对Sb的亲和力强,比表面积大,水氧化后对Sb的吸附能力增强。黄铁矿的水氧化对环境中Sb的地球化学循环和存在有很大影响。该研究为评估锑的环境风险和制定污染控制措施奠定了坚实的基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号