Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-07-23 , DOI: 10.1016/j.bbamem.2020.183417 Andrey S Kuznetsov 1 , Miftakh F Zamaletdinov 2 , Yaroslav V Bershatsky 3 , Anatoly S Urban 3 , Olga V Bocharova 3 , Amar Bennasroune 4 , Pascal Maurice 4 , Eduard V Bocharov 3 , Roman G Efremov 1
|
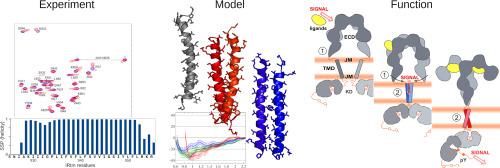
Despite the biological significance of insulin signaling, the molecular mechanisms of activation of the insulin receptor (IR) and other proteins from its family remain elusive. Current hypothesis on signal transduction suggests ligand-triggered structural changes in the extracellular domain followed by transmembrane (TM) domains closure and dimerization leading to trans-autophosphorylation and kinase activity in intracellular segments of the receptor. Using NMR spectroscopy, we detected dimerization of isolated TM segments of IR in different membrane-mimicking environments and observed multiple signals of NH groups of protein backbone possibly corresponding to several dimer conformations. Taking available experimental data as constraints, several atomistic models of dimeric TM domains of IR and insulin-like growth factor 1 (IGF-1R) receptors were elaborated. Molecular dynamics simulations of IR ectodomain revealed noticeable collective movements potentially responsible for closure of the C-termini of FnIII-3 domains and spatial approaching of TM helices upon insulin-induced receptor activation. In addition, we demonstrated that the intracellular part of the receptor does not impose restrictions on the positioning of TM helices in the membrane. Finally, we used two independent structure prediction methods to generate a series of dimer conformations followed by their cluster analysis and dimerization free energy estimation to select the best dimer models. Biological relevance of the later was further tested via comparison of the hydrophobic organization of TM helices for both wild-type receptors and their mutants. Based on these data, the ability of several segments from other proteins to functionally replace IR and/or IGF-1R TM domains was explained.
中文翻译:

胰岛素和IGF-1R受体跨膜结构域的二聚状态:结构和激活中的可能作用。
尽管胰岛素信号传导具有生物学意义,但激活胰岛素受体(IR)和其家族其他蛋白的分子机制仍然难以捉摸。关于信号转导的当前假设表明,胞外域中配体触发的结构变化随后是跨膜(TM)域关闭和二聚化,从而导致受体细胞内区段中的反式自磷酸化和激酶活性。使用NMR光谱,我们检测到在不同的膜模拟环境中IR的孤立TM片段的二聚化,并观察到了可能与几个二聚体构象相对应的蛋白质骨架NH基团的多个信号。以可用的实验数据为约束,阐述了IR和胰岛素样生长因子1(IGF-1R)受体的二聚TM域的几个原子模型。IR胞外域的分子动力学模拟表明,显着的集体运动可能导致FnIII-3域C末端的关闭以及胰岛素诱导的受体激活后TM螺旋的空间接近。另外,我们证明了受体的细胞内部分不对膜中TM螺旋的定位施加限制。最后,我们使用两种独立的结构预测方法来生成一系列二聚体构象,然后进行聚类分析和二聚自由能估计,以选择最佳的二聚体模型。通过比较TM螺旋对于野生型受体及其突变体的疏水性组织,进一步测试了后者的生物学相关性。基于这些数据,说明了其他蛋白质的多个片段在功能上替代IR和/或IGF-1R TM结构域的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号