当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydration of a 2D supra-molecular assembly: bitartrate on Cu(110)
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-21 , DOI: 10.1021/jacs.0c04747 Chenfang Lin 1 , George R Darling 1 , Matthew Forster 1 , Fiona McBride 1 , Alan Massey 1 , Andrew Hodgson 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-21 , DOI: 10.1021/jacs.0c04747 Chenfang Lin 1 , George R Darling 1 , Matthew Forster 1 , Fiona McBride 1 , Alan Massey 1 , Andrew Hodgson 1
Affiliation
|
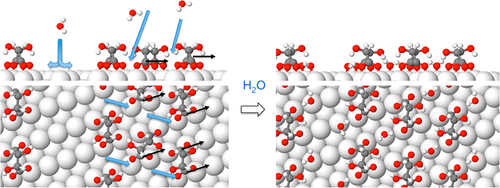
Hydration layers play a key role in many technical and biological systems, but our understanding of these structures remains very limited. Here, we investigate the molecular processes driving hydration of a chiral metal–organic surface, bitartrate on Cu(110), which consists of hydrogen-bonded bitartrate rows separated by exposed Cu. Initially water decorates the metal channels, hydrogen bonding to the exposed O ligands that bind bitartrate to Cu, but does not wet the bitartrate rows. At higher temperature, water inserts into the structure, breaks the existing intermolecular hydrogen bonds, and changes the adsorption site and footprint. Calculations show this process is driven by the creation of stable adsorption sites between the carboxylate ligands, to allow hydration of O–Cu ligands within the interior of the structure. This work suggests that hydration of polar metal–adsorbate ligands will be a dominant factor in many systems during surface hydration or self-assembly from solution.
中文翻译:

二维超分子组装体的水合:Cu(110) 上的酒石酸氢盐
水合层在许多技术和生物系统中发挥着关键作用,但我们对这些结构的了解仍然非常有限。在这里,我们研究了驱动手性金属有机表面水合的分子过程,即 Cu(110) 上的酒石酸氢盐,它由氢键合的酒石酸氢盐行组成,由暴露的铜分隔。最初,水装饰金属通道,氢键与暴露的 O 配体结合,将酒石酸氢盐与铜结合,但不会润湿酒石酸氢盐行。在较高温度下,水会插入结构中,破坏现有的分子间氢键,并改变吸附位点和足迹。计算表明,该过程是由羧酸盐配体之间产生稳定的吸附位点驱动的,以允许结构内部的 O-Cu 配体水合。
更新日期:2020-07-21
中文翻译:

二维超分子组装体的水合:Cu(110) 上的酒石酸氢盐
水合层在许多技术和生物系统中发挥着关键作用,但我们对这些结构的了解仍然非常有限。在这里,我们研究了驱动手性金属有机表面水合的分子过程,即 Cu(110) 上的酒石酸氢盐,它由氢键合的酒石酸氢盐行组成,由暴露的铜分隔。最初,水装饰金属通道,氢键与暴露的 O 配体结合,将酒石酸氢盐与铜结合,但不会润湿酒石酸氢盐行。在较高温度下,水会插入结构中,破坏现有的分子间氢键,并改变吸附位点和足迹。计算表明,该过程是由羧酸盐配体之间产生稳定的吸附位点驱动的,以允许结构内部的 O-Cu 配体水合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号