当前位置:
X-MOL 学术
›
Energy Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of Electrolyte for Vanadium Redox‐Flow Batteries Based on Vanadium Pentoxide
Energy Technology ( IF 3.6 ) Pub Date : 2020-07-21 , DOI: 10.1002/ente.202000522 Jan Martin 1 , Katharina Schafner 1 , Thomas Turek 1
Energy Technology ( IF 3.6 ) Pub Date : 2020-07-21 , DOI: 10.1002/ente.202000522 Jan Martin 1 , Katharina Schafner 1 , Thomas Turek 1
Affiliation
|
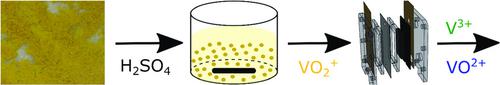
The vanadium redox‐flow battery is a promising technology for stationary energy storage. A reduction in system costs is essential for competitiveness with other chemical energy storage systems. A large share of costs is currently attributed to the electrolyte, which can be significantly reduced by production based on vanadium pentoxide (V2O5). In this study, the dissolution kinetics of V2O5 in diluted sulfuric acid and commercial vanadium electrolyte (VE) is determined. The low solubility of V2O5 in sulfuric acid can be overcome by partially using VE with a state of charge of −50% as solvent. In this way, a complete dissolution of V2O5 is possible within ≈10 min to achieve the desired vanadium concentration of 1.6 mol L−1. Moreover, the electrochemical reduction of an electrolyte containing VO2+ coupled with the oxygen evolution reaction at the anode is investigated. For these consecutive steps, an electrical energy demand of 1.69 kWh kg−1 is required to reach a state of charge of −50%. Finally, both processes are integrated into a plant concept for continuous electrolyte production.
中文翻译:

基于五氧化二钒的钒还原流电池电解液的制备
钒氧化还原液流电池是用于固定式能量存储的有前途的技术。降低系统成本对于与其他化学能源存储系统的竞争力至关重要。当前,大部分成本归因于电解质,通过基于五氧化二钒(V 2 O 5)的生产可以显着降低成本。在这项研究中,确定了V 2 O 5在稀硫酸和市售钒电解质(VE)中的溶解动力学。V 2 O 5在硫酸中的低溶解度可通过部分使用电荷状态为-50%的VE作为溶剂来克服。这样,V 2 O 5完全溶解在约10分钟内可能达到1.6 mol L -1的所需钒浓度。而且,研究了含VO 2 +的电解质的电化学还原以及在阳极的氧释放反应。对于这些连续步骤,需要达到1.69 kWh kg -1的电能才能达到-50%的充电状态。最后,这两个过程都被整合到一个工厂的概念中,以连续生产电解质。
更新日期:2020-09-05
中文翻译:

基于五氧化二钒的钒还原流电池电解液的制备
钒氧化还原液流电池是用于固定式能量存储的有前途的技术。降低系统成本对于与其他化学能源存储系统的竞争力至关重要。当前,大部分成本归因于电解质,通过基于五氧化二钒(V 2 O 5)的生产可以显着降低成本。在这项研究中,确定了V 2 O 5在稀硫酸和市售钒电解质(VE)中的溶解动力学。V 2 O 5在硫酸中的低溶解度可通过部分使用电荷状态为-50%的VE作为溶剂来克服。这样,V 2 O 5完全溶解在约10分钟内可能达到1.6 mol L -1的所需钒浓度。而且,研究了含VO 2 +的电解质的电化学还原以及在阳极的氧释放反应。对于这些连续步骤,需要达到1.69 kWh kg -1的电能才能达到-50%的充电状态。最后,这两个过程都被整合到一个工厂的概念中,以连续生产电解质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号