当前位置:
X-MOL 学术
›
Thermochim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermokinetic Parameters Evaluation Using Reaction Calorimetry: Application to Butyl Methacrylate Solution Radical Polymerization
Thermochimica Acta ( IF 3.1 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.tca.2020.178730 Isabelle Lahoud , Laurent Balland , Nicolas Brodu , Imed Ben Talouba , Nordine Mouhab , Catherine Legrand
Thermochimica Acta ( IF 3.1 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.tca.2020.178730 Isabelle Lahoud , Laurent Balland , Nicolas Brodu , Imed Ben Talouba , Nordine Mouhab , Catherine Legrand
|
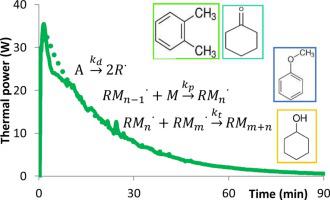
Abstract This study investigates the possibility to use reaction calorimetry to estimate thermokinetic parameters of an industrial reaction. The synthesis chosen in this study is the radical polymerization of butyl methacrylate in different organic solvents. The kinetic parameters estimation method is based on the comparison of experimental thermal power profiles released during polymerization and measured using a reaction calorimeter RC1-RTCal with calculated power profiles by means of a simplified model. Individual kinetic parameters of the three main steps (initiation, propagation, termination) were estimated using a minimization method based on a genetic algorithm. Contrary to classical methods, in this paper the three kinetic parameters are estimated simultaneously and at high conversion. The measurement of physical properties, average molecular mass, polydispersity index and viscosity, made it possible to understand the effect of the solvents nature on individual kinetic rate constants. Estimated results are in good consistency with those encountered in the literature obtained by classical methods.
中文翻译:

使用反应量热法评估热动力学参数:在甲基丙烯酸丁酯溶液自由基聚合中的应用
摘要 本研究探讨了使用反应量热法估算工业反应热动力学参数的可能性。本研究中选择的合成是甲基丙烯酸丁酯在不同有机溶剂中的自由基聚合。动力学参数估计方法基于聚合过程中释放的实验热功率曲线和使用反应量热仪 RC1-RTCal 测量的热功率曲线与通过简化模型计算的功率曲线的比较。使用基于遗传算法的最小化方法估计三个主要步骤(起始、传播、终止)的各个动力学参数。与经典方法相反,在本文中,三个动力学参数是在高转化率下同时估算的。物理性质的测量,平均分子量、多分散指数和粘度,使得了解溶剂性质对个体动力学速率常数的影响成为可能。估计结果与通过经典方法获得的文献中遇到的结果具有良好的一致性。
更新日期:2020-09-01
中文翻译:

使用反应量热法评估热动力学参数:在甲基丙烯酸丁酯溶液自由基聚合中的应用
摘要 本研究探讨了使用反应量热法估算工业反应热动力学参数的可能性。本研究中选择的合成是甲基丙烯酸丁酯在不同有机溶剂中的自由基聚合。动力学参数估计方法基于聚合过程中释放的实验热功率曲线和使用反应量热仪 RC1-RTCal 测量的热功率曲线与通过简化模型计算的功率曲线的比较。使用基于遗传算法的最小化方法估计三个主要步骤(起始、传播、终止)的各个动力学参数。与经典方法相反,在本文中,三个动力学参数是在高转化率下同时估算的。物理性质的测量,平均分子量、多分散指数和粘度,使得了解溶剂性质对个体动力学速率常数的影响成为可能。估计结果与通过经典方法获得的文献中遇到的结果具有良好的一致性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号