当前位置:
X-MOL 学术
›
Nano Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radiofrequency-responsive dual-valent gold nanoclusters for enhancing synergistic therapy of tumor ablation and artery embolization
Nano Today ( IF 13.2 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.nantod.2020.100934 Ling Li , Xiaopeng Guo , Xiaole Peng , Hongsen Zhang , Yiming Liu , Han Li , Xiaojun He , Dingwen Shi , Bin Xiong , Yanbing Zhao , Chuansheng Zheng , Xiangliang Yang
Nano Today ( IF 13.2 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.nantod.2020.100934 Ling Li , Xiaopeng Guo , Xiaole Peng , Hongsen Zhang , Yiming Liu , Han Li , Xiaojun He , Dingwen Shi , Bin Xiong , Yanbing Zhao , Chuansheng Zheng , Xiangliang Yang
|
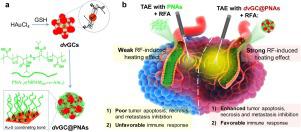
Abstract Interventional cancer therapy, till date, experiences a major challenge to improve the synergistic effect of radiofrequency ablation (RFA) and trans-artery embolization (TAE). Here, RF-responsive dual-valent gold nanoclusters (dvGC@PNAs), as multifunctional blood-vessel-embolic agents, have been successfully manufactured for comprehensive interventional theranostics of solid tumors. Distinct RF-induced heating effect was obtained by reducing Au(I)-thiolate complex using l -glutathione under the template polymer of temperature-sensitive poly(N-isopropylamide-co-acrylic acid) (PNAs), the well-defined dvGC@PNAs with the core-shell nanostructure of Au(0) atoms surrounded by a high content of Au(I) ions, thereby triggering cell apoptosis, necrosis, and metastasis inhibition. Efficient TAE cancer therapy can also be achieved by rapid diffusion of dvGC@PNAs into tumor peripheral arteries that impede tumor blood supply by their favorable temperature-sensitive sol-gel transition. The immunofluorescent analyses substantiated the greatly improved tumor microenvironment post TAE procedure, owing to the radiofrequency-responsive dvGC@PNAs, induced a favorable immune response, thereby augmenting the synergistic antitumor effect of RFA and TAE. The present study provides a valuable paradigm for ameliorating the synergistic interventional therapies by the rational design of RF-responsive metal nanoclusters.
中文翻译:

射频响应双价金纳米团簇用于增强肿瘤消融和动脉栓塞的协同治疗
摘要 迄今为止,介入性癌症治疗在提高射频消融 (RFA) 和经动脉栓塞 (TAE) 的协同效应方面面临重大挑战。在这里,射频响应双价金纳米团簇(dvGC@PNA)作为多功能血管栓塞剂,已成功制造用于实体瘤的综合介入治疗。通过在温度敏感的聚(N-异丙基酰胺-共聚-丙烯酸)(PNA)模板聚合物下使用 l-谷胱甘肽还原金(I)-硫醇盐复合物,获得了独特的射频诱导加热效果,定义明确的 dvGC@具有 Au(0) 原子核壳纳米结构的 PNA,被高含量的 Au(I) 离子包围,从而引发细胞凋亡、坏死和转移抑制。有效的 TAE 癌症治疗也可以通过 dvGC@PNA 快速扩散到肿瘤外周动脉中来实现,这些外周动脉通过其有利的温度敏感性溶胶-凝胶转变阻碍肿瘤血液供应。免疫荧光分析证实了 TAE 手术后大大改善的肿瘤微环境,由于射频响应 dvGC@PNA,诱导了有利的免疫反应,从而增强了 RFA 和 TAE 的协同抗肿瘤作用。本研究为通过射频响应金属纳米团簇的合理设计改善协同介入治疗提供了一个有价值的范例。免疫荧光分析证实了 TAE 手术后大大改善的肿瘤微环境,由于射频响应 dvGC@PNA,诱导了有利的免疫反应,从而增强了 RFA 和 TAE 的协同抗肿瘤作用。本研究为通过射频响应金属纳米团簇的合理设计改善协同介入治疗提供了一个有价值的范例。免疫荧光分析证实了 TAE 手术后大大改善的肿瘤微环境,由于射频响应 dvGC@PNA,诱导了有利的免疫反应,从而增强了 RFA 和 TAE 的协同抗肿瘤作用。本研究为通过射频响应金属纳米团簇的合理设计改善协同介入治疗提供了一个有价值的范例。
更新日期:2020-12-01
中文翻译:

射频响应双价金纳米团簇用于增强肿瘤消融和动脉栓塞的协同治疗
摘要 迄今为止,介入性癌症治疗在提高射频消融 (RFA) 和经动脉栓塞 (TAE) 的协同效应方面面临重大挑战。在这里,射频响应双价金纳米团簇(dvGC@PNA)作为多功能血管栓塞剂,已成功制造用于实体瘤的综合介入治疗。通过在温度敏感的聚(N-异丙基酰胺-共聚-丙烯酸)(PNA)模板聚合物下使用 l-谷胱甘肽还原金(I)-硫醇盐复合物,获得了独特的射频诱导加热效果,定义明确的 dvGC@具有 Au(0) 原子核壳纳米结构的 PNA,被高含量的 Au(I) 离子包围,从而引发细胞凋亡、坏死和转移抑制。有效的 TAE 癌症治疗也可以通过 dvGC@PNA 快速扩散到肿瘤外周动脉中来实现,这些外周动脉通过其有利的温度敏感性溶胶-凝胶转变阻碍肿瘤血液供应。免疫荧光分析证实了 TAE 手术后大大改善的肿瘤微环境,由于射频响应 dvGC@PNA,诱导了有利的免疫反应,从而增强了 RFA 和 TAE 的协同抗肿瘤作用。本研究为通过射频响应金属纳米团簇的合理设计改善协同介入治疗提供了一个有价值的范例。免疫荧光分析证实了 TAE 手术后大大改善的肿瘤微环境,由于射频响应 dvGC@PNA,诱导了有利的免疫反应,从而增强了 RFA 和 TAE 的协同抗肿瘤作用。本研究为通过射频响应金属纳米团簇的合理设计改善协同介入治疗提供了一个有价值的范例。免疫荧光分析证实了 TAE 手术后大大改善的肿瘤微环境,由于射频响应 dvGC@PNA,诱导了有利的免疫反应,从而增强了 RFA 和 TAE 的协同抗肿瘤作用。本研究为通过射频响应金属纳米团簇的合理设计改善协同介入治疗提供了一个有价值的范例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号