当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amyloid aggregation of spin-labeled β-lactoglobulin. Part I: Influence of spin labeling on amyloid aggregation
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.foodhyd.2020.106178 Jacqueline Lux , Timon R. Heyn , Ingo Kampen , Karin Schwarz , Julia K. Keppler , Anja Steffen-Heins
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.foodhyd.2020.106178 Jacqueline Lux , Timon R. Heyn , Ingo Kampen , Karin Schwarz , Julia K. Keppler , Anja Steffen-Heins
|
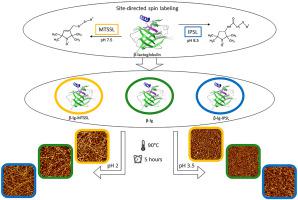
Abstract Site-directed spin labeling (SDSL) of the natural food protein β-lactoglobulin (β-lg) was established with the aim of characterizing amyloid aggregation while explicitly avoiding the usual manipulation of primary protein structure. For its successful application, spin labels must not alter secondary protein structure or the formation of β-lg amyloid aggregates. The two spin labels—the MTSSL (flexible S–S binding) and Iodoacetamido-proxyl spin label (IPSL) (more rigid C–S binding)—were used for amyloid aggregation at pH 2 and pH 3.5. At pH 3.5, IPSL caused minor changes in the secondary protein structure, where it reduced intra- and intermolecular β-sheets as determined by ATR-FTIR. Analysis of the extent of amyloid aggregation using thioflavin T fluorescence indicated that the spin probes interfered with the binding of the fluorescent probes to the β sheets. Non-amyloid and amyloid fractions were obtained from the amyloid aggregated system by ultrafiltration (300 kDa), which also proved to be equivalent and independent of the spin labeling process. Atomic force microscopy and size-exclusion chromatography results suggest the same building blocks and morphologies between unlabeled and spin-labeled proteins; therefore, substantial changes in the behavior of β-lg during amyloid aggregation can be excluded. Ultimately, 10–17% of the β-lg molecules were labeled so that the SDSL approach can be used to label the natural food protein at these low concentrations without affecting protein conformation or the formation of amyloid aggregates, which may subsequently provide deep insights into the aggregation mechanism of food proteins under processing conditions.
中文翻译:

自旋标记的 β-乳球蛋白的淀粉样蛋白聚集。第一部分:自旋标记对淀粉样蛋白聚集的影响
摘要 天然食物蛋白 β-乳球蛋白 (β-lg) 的定点自旋标记 (SDSL) 旨在表征淀粉样蛋白聚集,同时明确避免对主要蛋白质结构的通常操作。为了成功应用,自旋标记不得改变二级蛋白质结构或 β-lg 淀粉样蛋白聚集体的形成。两种自旋标记——MTSSL(灵活的 S-S 结合)和碘乙酰胺-代理自旋标记 (IPSL)(更刚性的 C-S 结合)——用于在 pH 2 和 pH 3.5 下进行淀粉样蛋白聚集。在 pH 3.5 时,IPSL 会导致二级蛋白质结构发生微小变化,从而减少了 ATR-FTIR 确定的分子内和分子间 β-折叠。使用硫代黄素 T 荧光分析淀粉样蛋白聚集的程度表明自旋探针干扰了荧光探针与 β 片层的结合。通过超滤 (300 kDa) 从淀粉样蛋白聚集系统中获得非淀粉样蛋白和淀粉样蛋白部分,这也被证明是等效的且独立于自旋标记过程。原子力显微镜和尺寸排阻色谱结果表明未标记和自旋标记的蛋白质之间具有相同的构建块和形态;因此,可以排除淀粉样蛋白聚集过程中 β-lg 行为的重大变化。最终,
更新日期:2021-03-01
中文翻译:

自旋标记的 β-乳球蛋白的淀粉样蛋白聚集。第一部分:自旋标记对淀粉样蛋白聚集的影响
摘要 天然食物蛋白 β-乳球蛋白 (β-lg) 的定点自旋标记 (SDSL) 旨在表征淀粉样蛋白聚集,同时明确避免对主要蛋白质结构的通常操作。为了成功应用,自旋标记不得改变二级蛋白质结构或 β-lg 淀粉样蛋白聚集体的形成。两种自旋标记——MTSSL(灵活的 S-S 结合)和碘乙酰胺-代理自旋标记 (IPSL)(更刚性的 C-S 结合)——用于在 pH 2 和 pH 3.5 下进行淀粉样蛋白聚集。在 pH 3.5 时,IPSL 会导致二级蛋白质结构发生微小变化,从而减少了 ATR-FTIR 确定的分子内和分子间 β-折叠。使用硫代黄素 T 荧光分析淀粉样蛋白聚集的程度表明自旋探针干扰了荧光探针与 β 片层的结合。通过超滤 (300 kDa) 从淀粉样蛋白聚集系统中获得非淀粉样蛋白和淀粉样蛋白部分,这也被证明是等效的且独立于自旋标记过程。原子力显微镜和尺寸排阻色谱结果表明未标记和自旋标记的蛋白质之间具有相同的构建块和形态;因此,可以排除淀粉样蛋白聚集过程中 β-lg 行为的重大变化。最终,











































 京公网安备 11010802027423号
京公网安备 11010802027423号