Chem ( IF 19.1 ) Pub Date : 2020-07-22 , DOI: 10.1016/j.chempr.2020.06.035 Ayan Dasgupta , Rasool Babaahmadi , Ben Slater , Brian F. Yates , Alireza Ariafard , Rebecca L. Melen
|
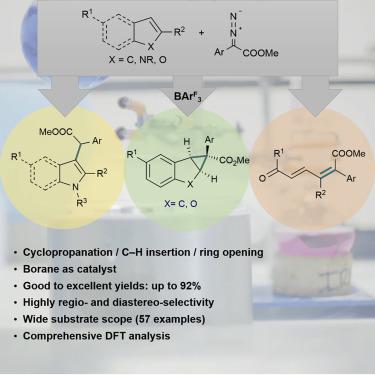
Lewis acidic boranes have been shown to be effective metal-free catalysts for highly selective reactions of donor-acceptor diazo compounds to a range of substrates. The reactions of α-aryl α-diazoesters with nitrogen heterocycles indole or pyrrole selectively generate C3 and C2 C–H insertion products, respectively, in good to excellent yields even when using unprotected indoles. Alternatively, benzofuran, indene, and alkene substrates give exclusively cyclopropanated products with α-aryl α-diazoesters, whereas the reactions with furans lead to ring-opening. Comprehensive theoretical calculations have been used to explain the differing reactivities and high selectivities of these reactions. Overall, this work demonstrates the selective metal-free catalytic reactions of α-aryl α-diazoesters with (hetero)cycles and alkenes. This simple, mild reaction protocol represents an alternative to the commonly used precious metal systems and may provide future applications in the generation of biologically active compounds.
中文翻译:

硼烷催化的立体选择性C–H插入,环丙烷化和开环反应
已经证明路易斯酸性硼烷是供体-受体重氮化合物与一系列底物高度选择性反应的有效无金属催化剂。α-芳基α-重氮酸酯与氮杂环吲哚或吡咯的反应分别选择性地生成C3和C2 C–H插入产物,即使使用未保护的吲哚,其收率也良好至优异。备选地,苯并呋喃,茚和烯烃底物仅产生具有α-芳基α-二重氮酯的环丙烷化产物,而与呋喃的反应导致开环。全面的理论计算已用于解释这些反应的不同反应性和高选择性。总的来说,这项工作证明了α-芳基α-重氮酸酯与(杂)环和烯烃的选择性无金属催化反应。这个简单











































 京公网安备 11010802027423号
京公网安备 11010802027423号