当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single-Step Conversion of Ethanol to n-Butene over Ag-ZrO2/SiO2 Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-07-20 , DOI: 10.1021/acscatal.0c02235 Vanessa Lebarbier Dagle 1 , Austin D. Winkelman 1, 2 , Nicholas R. Jaegers 1, 2 , Johnny Saavedra-Lopez 1 , Jianzhi Hu 1 , Mark H. Engelhard 3 , Susan E. Habas 4 , Sneha A. Akhade 1, 5 , Libor Kovarik 1 , Vassilliki-Alexandra Glezakou 1 , Roger Rousseau 1 , Yong Wang 1, 2 , Robert A. Dagle 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-07-20 , DOI: 10.1021/acscatal.0c02235 Vanessa Lebarbier Dagle 1 , Austin D. Winkelman 1, 2 , Nicholas R. Jaegers 1, 2 , Johnny Saavedra-Lopez 1 , Jianzhi Hu 1 , Mark H. Engelhard 3 , Susan E. Habas 4 , Sneha A. Akhade 1, 5 , Libor Kovarik 1 , Vassilliki-Alexandra Glezakou 1 , Roger Rousseau 1 , Yong Wang 1, 2 , Robert A. Dagle 1
Affiliation
|
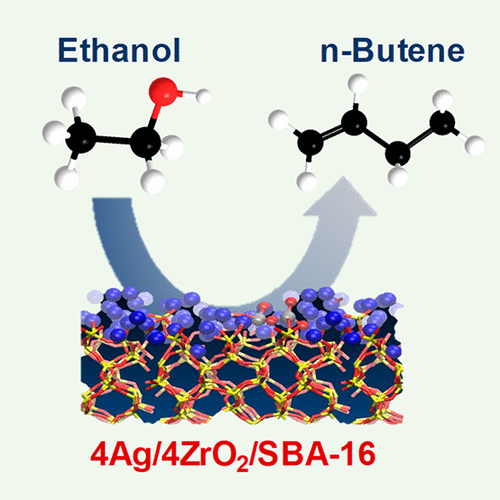
Ethanol is a promising platform molecule for production of a variety of fuels and chemicals. Of particular interest is the production of middle distillate fuels (i.e., jet and diesel blendstock) from renewable ethanol feedstock. State-of-the-art alcohol-to-jet technology requires multiple process steps based on the catalytic dehydration of ethanol to ethylene, followed by a multistep oligomerization including n-butene formation and then hydrotreatment and distillation. Here we report that, over Ag-ZrO2/SBA-16 with balanced metal and Lewis acid sites, ethanol is directly converted to n-butene (1- and 2-butene mixtures) with an exceptional butene-rich olefin selectivity of 88% at 99% conversion. The need for the ethanol dehydration to ethylene step is eliminated. Thus, it offers the potential for a reduction in the number of required processing units versus conventional alcohol-to-jet technology. We also found that the C4 product distribution, n-butene and/or 1,3-butadiene, can be tailored on this catalyst by tuning the hydrogen feed partial pressure and other process/catalyst parameters. With sufficient hydrogen partial pressure, 1,3-butadiene is completely and selectively hydrogenated to form n-butene. The reaction mechanism was elucidated through operando-nuclear magnetic resonance investigations coupled with reactivity measurements. Ethanol is first dehydrogenated to acetaldehyde over the metallic Ag, then acetaldehyde is converted to crotonaldehyde over the acid sites of ZrO2/SiO2 via aldol condensation followed by dehydration. This is followed by a Meerwein–Ponndorf–Verley reduction of crotonaldehyde to butadiene intermediate that is hydrogenated into n-butene over metallic Ag and ZrO2. A minor fraction of n-butene is also produced from crotonaldehyde reduction to butyraldehyde instead of butadiene. Isotopically labeled ethanol NMR experiments demonstrated that ethanol, rather than H2, is the source of H for the hydrogenation of crotonaldehyde to butyraldehyde. Combined experimental-computational investigation reveals how changes in silver and zirconium composition and the silver oxidation state affects reactivity under controlled hydrogen partial pressures and after prolonged run times. Finally, catalyst effectiveness also was demonstrated when using wet ethanol feed, thus highlighting process flexibility in terms of feedstock purity requirements.
中文翻译:

Ag-ZrO 2 / SiO 2催化剂将乙醇一步转化为正丁烯
乙醇是用于生产各种燃料和化学品的有前途的平台分子。特别令人感兴趣的是由可再生乙醇原料生产中间馏分燃料(即喷气和柴油混合原料)。最先进的酒精喷射技术需要基于乙醇催化脱水为乙烯的多重工艺步骤,然后进行多步低聚反应,包括形成正丁烯,然后进行加氢处理和蒸馏。在这里,我们报告说,在具有平衡金属和路易斯酸位点的Ag-ZrO 2 / SBA-16上,乙醇直接转化为n-丁烯(1-和2-丁烯混合物),在99%的转化率下具有88%的优异的富丁烯烯烃选择性。消除了乙醇脱水成乙烯步骤的需要。因此,与传统的酒精喷射技术相比,它具有减少所需处理单元数量的潜力。我们还发现,可以通过调节氢气进料的分压和其他工艺/催化剂参数,在该催化剂上调整正丁烯和/或1,3-丁二烯的C 4产物分布。在足够的氢分压下,1,3-丁二烯被完全选择性地氢化形成n-丁烯。通过操作核磁共振研究以及反应性测量来阐明反应机理。首先将乙醇在金属Ag上脱氢为乙醛,然后在乙醛上通过醛醇缩合在ZrO 2 / SiO 2的酸性部位上转化为巴豆醛,然后进行脱水。随后是Meerwein-Ponndorf-Verley将巴豆醛还原为丁二烯中间体,然后在金属Ag和ZrO 2上将其氢化为正丁烯。从巴豆醛还原成丁醛而不是丁二烯也产生了少量的正丁烯。同位素标记的乙醇NMR实验表明,乙醇而不是H2是巴豆醛加氢成丁醛的H的来源。组合的实验计算研究表明,在受控的氢气分压和长时间运行后,银和锆组成的变化以及银的氧化态如何影响反应性。最后,当使用湿乙醇进料时,也证明了催化剂的有效性,因此突出了工艺上对原料纯度要求的灵活性。
更新日期:2020-09-20
中文翻译:

Ag-ZrO 2 / SiO 2催化剂将乙醇一步转化为正丁烯
乙醇是用于生产各种燃料和化学品的有前途的平台分子。特别令人感兴趣的是由可再生乙醇原料生产中间馏分燃料(即喷气和柴油混合原料)。最先进的酒精喷射技术需要基于乙醇催化脱水为乙烯的多重工艺步骤,然后进行多步低聚反应,包括形成正丁烯,然后进行加氢处理和蒸馏。在这里,我们报告说,在具有平衡金属和路易斯酸位点的Ag-ZrO 2 / SBA-16上,乙醇直接转化为n-丁烯(1-和2-丁烯混合物),在99%的转化率下具有88%的优异的富丁烯烯烃选择性。消除了乙醇脱水成乙烯步骤的需要。因此,与传统的酒精喷射技术相比,它具有减少所需处理单元数量的潜力。我们还发现,可以通过调节氢气进料的分压和其他工艺/催化剂参数,在该催化剂上调整正丁烯和/或1,3-丁二烯的C 4产物分布。在足够的氢分压下,1,3-丁二烯被完全选择性地氢化形成n-丁烯。通过操作核磁共振研究以及反应性测量来阐明反应机理。首先将乙醇在金属Ag上脱氢为乙醛,然后在乙醛上通过醛醇缩合在ZrO 2 / SiO 2的酸性部位上转化为巴豆醛,然后进行脱水。随后是Meerwein-Ponndorf-Verley将巴豆醛还原为丁二烯中间体,然后在金属Ag和ZrO 2上将其氢化为正丁烯。从巴豆醛还原成丁醛而不是丁二烯也产生了少量的正丁烯。同位素标记的乙醇NMR实验表明,乙醇而不是H2是巴豆醛加氢成丁醛的H的来源。组合的实验计算研究表明,在受控的氢气分压和长时间运行后,银和锆组成的变化以及银的氧化态如何影响反应性。最后,当使用湿乙醇进料时,也证明了催化剂的有效性,因此突出了工艺上对原料纯度要求的灵活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号