当前位置:
X-MOL 学术
›
Biotechnol. Prog.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Promising insights into the kosmotropic effect of magnetic nanoparticles on proteins: The pivotal role of protein corona formation.
Biotechnology Progress ( IF 2.5 ) Pub Date : 2020-07-21 , DOI: 10.1002/btpr.3051 Reza Fattah 1 , Hamid Rashedi 1 , Fatemeh Yazdian 2 , Seyed Babak Mousavi 3 , Ahmad Fazeli 3, 4
Biotechnology Progress ( IF 2.5 ) Pub Date : 2020-07-21 , DOI: 10.1002/btpr.3051 Reza Fattah 1 , Hamid Rashedi 1 , Fatemeh Yazdian 2 , Seyed Babak Mousavi 3 , Ahmad Fazeli 3, 4
Affiliation
|
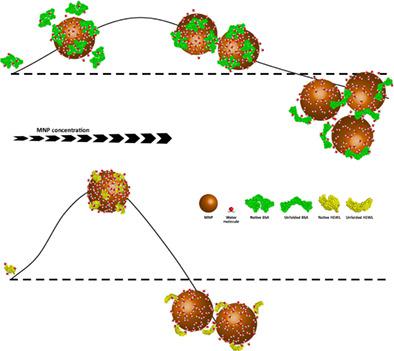
Increasing concerns about biosafety of nanoparticles (NPs) has raised the need for detailed knowledge of NP interactions with biological molecules especially proteins. Herein, the concentration‐dependent effect of magnetic NPs (MNPs) on bovine serum albumin and hen egg white lysozyme was explored. The X‐ray diffraction patterns, zeta potential, and dynamic light scattering measurements together with scanning electron microscopy images were employed to characterize MNPs synthesized through coprecipitation method. Then, we studied the behavior of two model proteins with different surface charges and structural properties on interaction with Fe3O4. A thorough investigation of protein–MNP interaction by the help of intrinsic fluorescence at different experimental conditions revealed that affinity of proteins for MNPs is strongly affected by the similarity of protein and MNP surface charges. MNPs exerted structure‐making kosmotropic effect on both proteins under a concentration threshold; however, binding strength was found to determine the extent of stabilizing effect as well as magnitude of the concentration threshold. Circular dichroism spectra showed that proteins with less resistance to conformational deformations are more prone to secondary structure changes upon adsorption on MNPs. By screening thermal aggregation of proteins in the presence of Fe3O4, it was also found that like chemical stability, thermal stability is influenced to a higher extent in more strongly bound proteins. Overall, this report not only provides an integrated picture of protein–MNP interaction but also sheds light on the molecular mechanism underling this process.
中文翻译:

关于磁性纳米粒子对蛋白质的亲液效应的有希望的见解:蛋白质电晕形成的关键作用。
对纳米颗粒 (NPs) 生物安全性的日益关注提出了对纳米颗粒与生物分子(尤其是蛋白质)相互作用的详细知识的需求。在此,研究了磁性纳米颗粒(MNPs)对牛血清白蛋白和鸡蛋清溶菌酶的浓度依赖性影响。X 射线衍射图、zeta 电位和动态光散射测量以及扫描电子显微镜图像被用来表征通过共沉淀法合成的 MNP。然后,我们研究了具有不同表面电荷和结构特性的两种模型蛋白质在与 Fe 3 O 4相互作用时的行为。. 在不同实验条件下借助内在荧光对蛋白质-MNP 相互作用的彻底研究表明,蛋白质对 MNP 的亲和力受蛋白质和 MNP 表面电荷相似性的强烈影响。MNPs 在浓度阈值下对两种蛋白质都发挥了结构形成的亲液作用;然而,发现结合强度可以确定稳定效果的程度以及浓度阈值的大小。圆二色光谱表明,对构象变形的抵抗力较小的蛋白质在吸附在 MNP 上时更容易发生二级结构变化。通过在 Fe 3 O 4存在下筛选蛋白质的热聚集还发现,与化学稳定性一样,热稳定性在结合力更强的蛋白质中受到更大程度的影响。总的来说,这份报告不仅提供了蛋白质-MNP 相互作用的综合图景,而且还阐明了这一过程背后的分子机制。
更新日期:2020-07-21
中文翻译:

关于磁性纳米粒子对蛋白质的亲液效应的有希望的见解:蛋白质电晕形成的关键作用。
对纳米颗粒 (NPs) 生物安全性的日益关注提出了对纳米颗粒与生物分子(尤其是蛋白质)相互作用的详细知识的需求。在此,研究了磁性纳米颗粒(MNPs)对牛血清白蛋白和鸡蛋清溶菌酶的浓度依赖性影响。X 射线衍射图、zeta 电位和动态光散射测量以及扫描电子显微镜图像被用来表征通过共沉淀法合成的 MNP。然后,我们研究了具有不同表面电荷和结构特性的两种模型蛋白质在与 Fe 3 O 4相互作用时的行为。. 在不同实验条件下借助内在荧光对蛋白质-MNP 相互作用的彻底研究表明,蛋白质对 MNP 的亲和力受蛋白质和 MNP 表面电荷相似性的强烈影响。MNPs 在浓度阈值下对两种蛋白质都发挥了结构形成的亲液作用;然而,发现结合强度可以确定稳定效果的程度以及浓度阈值的大小。圆二色光谱表明,对构象变形的抵抗力较小的蛋白质在吸附在 MNP 上时更容易发生二级结构变化。通过在 Fe 3 O 4存在下筛选蛋白质的热聚集还发现,与化学稳定性一样,热稳定性在结合力更强的蛋白质中受到更大程度的影响。总的来说,这份报告不仅提供了蛋白质-MNP 相互作用的综合图景,而且还阐明了这一过程背后的分子机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号