当前位置:
X-MOL 学术
›
Batteries Supercaps
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvation of NaPF6 in Diglyme Solution for Battery Electrolytes
Batteries & Supercaps ( IF 5.1 ) Pub Date : 2020-07-20 , DOI: 10.1002/batt.202000144 Anders C. S. Jensen 1, 2 , Heather Au 2 , Sabrina Gärtner 3 , Maria‐Magdalena Titirici 2 , Alan J. Drew 1
Batteries & Supercaps ( IF 5.1 ) Pub Date : 2020-07-20 , DOI: 10.1002/batt.202000144 Anders C. S. Jensen 1, 2 , Heather Au 2 , Sabrina Gärtner 3 , Maria‐Magdalena Titirici 2 , Alan J. Drew 1
Affiliation
|
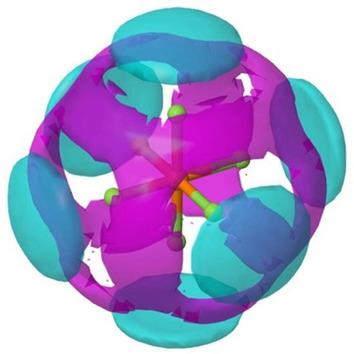
Glymes present a promising new group of electrolyte solvents for sodium‐ion batteries. Not only do they have excellent electrolyte solvent properties but they also enable the intercalation of sodium into graphite as sodium‐glyme complexes, a reaction which is not possible for sodium in conventional electrolyte solvents. However, little is known about the solution structure of these complexes, especially for sodium salts, and why glymes enable this process while other commonly used electrolyte solvents do not. Here, a combination of neutron total scattering and empirical potential structure refinement was used to characterize the solvent structure around the ions, for a NaPF6 solution in diglyme. This showed that 82 % of the sodium ions are bound as Na+(diglyme)2 complexes, the conformation needed for intercalation into graphite, with the rest forming various contact ion pairs. The model also showed that very weak hydrogen bonding interactions exist between the anion and the diglyme molecules.
中文翻译:

NaPF6在二甘醇二甲醚溶液中的溶解度
Glymes展示了一种有前途的新型钠离子电池电解质溶剂。它们不仅具有出色的电解质溶剂性能,而且还可以将钠作为钠-糖蛋白络合物嵌入石墨中,这是常规电解质溶剂中钠无法实现的反应。但是,对于这些络合物的溶液结构,尤其是钠盐的溶液结构,以及为何甘醇二甲醚能够实现此过程而其他常用电解质溶剂却无法做到这一点,人们知之甚少。在此,对于在二甘醇二甲醚中的NaPF 6溶液,结合了中子总散射和经验势能结构的精化来表征离子周围的溶剂结构。这表明82%的钠离子以Na +(二甘醇二甲醚)2的形式结合配合物,需要嵌入石墨中的构象,其余形成各种接触离子对。该模型还表明,阴离子与二甘醇二甲醚分子之间存在非常弱的氢键相互作用。
更新日期:2020-07-20
中文翻译:

NaPF6在二甘醇二甲醚溶液中的溶解度
Glymes展示了一种有前途的新型钠离子电池电解质溶剂。它们不仅具有出色的电解质溶剂性能,而且还可以将钠作为钠-糖蛋白络合物嵌入石墨中,这是常规电解质溶剂中钠无法实现的反应。但是,对于这些络合物的溶液结构,尤其是钠盐的溶液结构,以及为何甘醇二甲醚能够实现此过程而其他常用电解质溶剂却无法做到这一点,人们知之甚少。在此,对于在二甘醇二甲醚中的NaPF 6溶液,结合了中子总散射和经验势能结构的精化来表征离子周围的溶剂结构。这表明82%的钠离子以Na +(二甘醇二甲醚)2的形式结合配合物,需要嵌入石墨中的构象,其余形成各种接触离子对。该模型还表明,阴离子与二甘醇二甲醚分子之间存在非常弱的氢键相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号