Marine Chemistry ( IF 3.0 ) Pub Date : 2020-07-21 , DOI: 10.1016/j.marchem.2020.103858 Matthew R. Jones , George W. Luther , Bradley M. Tebo
|
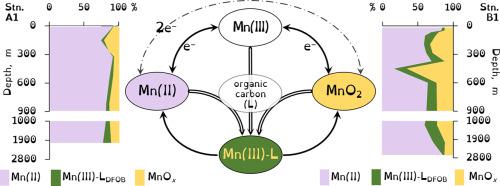
As manganese cycles between its three oxidation states, encompassing soluble and particulate phases, it influences the biogeochemistry of organic carbon, nutrients, and many trace elements. However, measurements of manganese distributions and speciation in the open ocean have typically been based only on differentiating soluble (assumed to be manganese(II)) and particulate (assumed to be manganese(III,IV) oxides) forms. We measured particulate oxidized manganese (MnOx, where x ≈ 1.8–2), reactive soluble manganese(III) (soluble manganese(III) that forms a complex with desferrioxamine-B (Mn(III)-LDFOB)), and total dissolved manganese (dMnT; manganese(II) + Mn(III)-LDFOB) in Northwest Atlantic offshore waters (10–2600 m). Mn(III)-LDFOB concentrations were from below the detection limit (0.008 nM) up to 0.76 nM and measured throughout the water column with higher concentrations near the base of the euphotic zone (~ 100 m), in the oxygen minimum zone (OMZ), and generally increasing from below the OMZ into the bottom waters. MnOx ranged from 0.19 nM to 3.52 nM in the water column. Concentrations were high near the base of the euphotic zone where reactions of MnOx with organic material are the likely source of the Mn(III)-LDFOB observed there. Elevated MnOx was also occasionally found in deep waters likely due to turbidity layers resulting from sediment resuspension. Mn(III)-LDFOB accounted for up to 45% of the dMnT and up to 74% of the total oxidized manganese (Mn(III)-LDFOB + MnOx) in different regions of the water column. Mn(III)-LDFOB contributed 10–20% of the generally uniform total dissolved manganese concentration in the deep ocean. Both soluble and particulate oxidized forms of manganese (Mn(III)-L and MnOx) are a significant component of the deep water manganese pool and likely play a prominent role in oceanic redox chemistry and organic carbon re-mineralization.
中文翻译:

西北大西洋中可溶性锰(II),可溶性反应性Mn(III)-L和颗粒状MnO 2的分布和浓度
锰在其三个氧化态(包括可溶相和颗粒相)之间循环时,会影响有机碳,养分和许多微量元素的生物地球化学。但是,测量公海中锰分布和形态的方法通常仅基于区分可溶形式(假定为锰(II))和颗粒状形式(假定为锰(III,IV)氧化物)。我们测得的微粒氧化锰(MnO的X,其中X ≈1.8-2),活性可溶性锰(III)(可溶性锰(III),其形成的复合物与去铁胺B(锰(III)-L DFOB)),并且总溶解锰(dMn T ;锰(II)+ Mn(III)-L DFOB)在西北大西洋近海水域(10–2600 m)。Mn(III)-L DFOB的浓度从检测限以下(0.008 nM)到0.76 nM,并且在整个水柱中均在富氧区底部(〜100 m)附近的最小氧气区中以较高的浓度进行测量( OMZ),并且通常从OMZ下方增加到底部水域。在水柱中,MnO x的范围为0.19 nM至3.52 nM。MnO x与有机材料的反应是在此处观察到的Mn(III)-L DFOB的可能来源,在富营养区底部附近的浓度很高。在深水中偶尔还会发现MnO x升高,这可能是由于沉积物重悬而形成的浑浊层所致。锰(III)-L在水柱的不同区域中,DFOB占dMn T的比例高达45%,占氧化锰总量的74%(Mn(III)-L DFOB + MnO x)。Mn(III)-L DFOB在深海中贡献了通常均匀的总溶解锰浓度的10-20%。锰(Mn(III)-L和MnO x)的可溶性和微粒氧化形式都是深水锰池的重要组成部分,并且可能在海洋氧化还原化学和有机碳再矿化中发挥重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号