当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Towards a sustainable generation of pseudopterosin-type bioactives
Green Chemistry ( IF 9.3 ) Pub Date : 2020-07-20 , DOI: 10.1039/d0gc01697g Marion Ringel 1, 2, 3, 4, 5 , Markus Reinbold 1, 2, 3, 4, 5 , Max Hirte 1, 2, 3, 4, 5 , Martina Haack 1, 2, 3, 4, 5 , Claudia Huber 2, 3, 5, 6, 7 , Wolfgang Eisenreich 2, 3, 5, 6, 7 , Mahmoud A. Masri 1, 2, 3, 4, 5 , Gerhard Schenk 8, 9, 10, 11 , Luke W. Guddat 8, 9, 10, 11 , Bernhard Loll 5, 12, 13, 14, 15 , Russell Kerr 16, 17, 18, 19, 20 , Daniel Garbe 1, 2, 3, 4, 5 , Thomas Brück 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2020-07-20 , DOI: 10.1039/d0gc01697g Marion Ringel 1, 2, 3, 4, 5 , Markus Reinbold 1, 2, 3, 4, 5 , Max Hirte 1, 2, 3, 4, 5 , Martina Haack 1, 2, 3, 4, 5 , Claudia Huber 2, 3, 5, 6, 7 , Wolfgang Eisenreich 2, 3, 5, 6, 7 , Mahmoud A. Masri 1, 2, 3, 4, 5 , Gerhard Schenk 8, 9, 10, 11 , Luke W. Guddat 8, 9, 10, 11 , Bernhard Loll 5, 12, 13, 14, 15 , Russell Kerr 16, 17, 18, 19, 20 , Daniel Garbe 1, 2, 3, 4, 5 , Thomas Brück 1, 2, 3, 4, 5
Affiliation
|
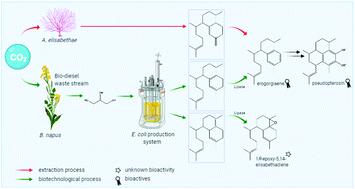
Pseudopterosins (Ps), marine diterpene glycosides derived from the marine octocoral Antillogorgia elisabethae, have potent anti-inflammatory activity demonstrated in phase II clinical trials. As multi-step total chemical synthesis is not economical, Ps applications are limited to anti-irritant cosmeceuticals, which are exclusively sourced by unsustainable coral extraction. While chemical intermediates in Ps biosynthesis have been resolved, the underlying biochemical processes remain elusive. Therefore, a coral independent route to enable sustainable access to Ps and respective bioactive precusors is required. Here, in silico guided mutagenesis of the hydropyrene synthase (HpS) from Streptomyces clavuligerus reveals five unique, catalytically relevant methionine residues, and affords selective formation of biosynthetic Ps precursors isoelisabethatriene A and B in an Escherichia coli host with total terpene yield of HpS M75L of 41.91 ± 1.87 mg L−1. This is the first experimental precedence of methionine residues being involved in terpene synthase catalysis, indicating that HpS may belong to a new subfamily. Further, lipase catalysed chemo-enzymatic oxidation differentially transforms the isomers isoelisabethatriene A and B to the advanced Ps precursor erogorgiaene (yield: 69%) and the new compound 1R-epoxy-elisabetha-5,14-diene (EED) (yield: 41%), respectively. As erogorgiaene has significant activity against multi-drug resistant Mycobacterium tuberculosis, the process provides a consolidated and scalable access to erogorgiaene, which allows further clinical development of this compound. Moreover, erogorgiaene access also provides a consolidated route for Ps synthesis. Synergistically EED generation affords a new scaffold for Ps-type drug development. These technologies assist in preserving fragile coral reef ecosystem biodiversity and open a fast track for clinical Ps development.
中文翻译:

迈向可持续发展的拟蝶呤类生物活性物质
假单胞菌素(Psudopterosins)(Ps),是源自海洋八倍体Antillogorgia elisabethae的海洋二萜糖苷,在II期临床试验中具有有效的抗炎活性。由于多步全化学合成是不经济的,因此Ps的应用仅限于抗刺激性的药妆品,这些药材仅来自不可持续的珊瑚提取。虽然已经解决了Ps生物合成中的化学中间体,但潜在的生化过程仍然难以捉摸。因此,需要一条独立于珊瑚的途径,以可持续获取Ps和各自的生物活性先兆。在这里,计算机控制的诱变链霉菌的水py合酶(HpS)的诱变揭示了五个独特的,催化相关的蛋氨酸残基,并在大肠杆菌宿主中选择性形成生物合成的Ps前体异elisabethatriene A和B ,HpS M75L的总萜烯收率为41.91±1.87 mg L -1。这是蛋氨酸残基参与萜烯合酶催化的第一个实验先例,表明HpS可能属于一个新的亚家族。此外,脂肪酶催化的化学酶氧化反应将异构体异伊利沙伯那来烯A和B差异转化为高级Ps前体erogorgiaene(收率:69%)和新化合物1 R-环氧-elisabetha-5,14-二烯(EED)(收率: 41%)。由于erogorgiaene具有抗多药耐药性的显着活性结核分枝杆菌,该过程提供了对erogorgiaene的整合和可扩展的访问,这使得该化合物的进一步临床开发成为可能。此外,erogorgiaene的访问也为Ps合成提供了整合的途径。协同产生EED为Ps型药物开发提供了新的支架。这些技术有助于保护脆弱的珊瑚礁生态系统生物多样性,并为临床Ps的发展打开一条捷径。
更新日期:2020-09-21
中文翻译:

迈向可持续发展的拟蝶呤类生物活性物质
假单胞菌素(Psudopterosins)(Ps),是源自海洋八倍体Antillogorgia elisabethae的海洋二萜糖苷,在II期临床试验中具有有效的抗炎活性。由于多步全化学合成是不经济的,因此Ps的应用仅限于抗刺激性的药妆品,这些药材仅来自不可持续的珊瑚提取。虽然已经解决了Ps生物合成中的化学中间体,但潜在的生化过程仍然难以捉摸。因此,需要一条独立于珊瑚的途径,以可持续获取Ps和各自的生物活性先兆。在这里,计算机控制的诱变链霉菌的水py合酶(HpS)的诱变揭示了五个独特的,催化相关的蛋氨酸残基,并在大肠杆菌宿主中选择性形成生物合成的Ps前体异elisabethatriene A和B ,HpS M75L的总萜烯收率为41.91±1.87 mg L -1。这是蛋氨酸残基参与萜烯合酶催化的第一个实验先例,表明HpS可能属于一个新的亚家族。此外,脂肪酶催化的化学酶氧化反应将异构体异伊利沙伯那来烯A和B差异转化为高级Ps前体erogorgiaene(收率:69%)和新化合物1 R-环氧-elisabetha-5,14-二烯(EED)(收率: 41%)。由于erogorgiaene具有抗多药耐药性的显着活性结核分枝杆菌,该过程提供了对erogorgiaene的整合和可扩展的访问,这使得该化合物的进一步临床开发成为可能。此外,erogorgiaene的访问也为Ps合成提供了整合的途径。协同产生EED为Ps型药物开发提供了新的支架。这些技术有助于保护脆弱的珊瑚礁生态系统生物多样性,并为临床Ps的发展打开一条捷径。









































 京公网安备 11010802027423号
京公网安备 11010802027423号