当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Tagetitoxin
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-20 , DOI: 10.1021/jacs.0c06641 Chi He 1 , Hang Chu 1 , Thomas P Stratton 1 , David Kossler 1 , Kelly J Eberle 1 , Dillon T Flood 1 , Phil S Baran 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-20 , DOI: 10.1021/jacs.0c06641 Chi He 1 , Hang Chu 1 , Thomas P Stratton 1 , David Kossler 1 , Kelly J Eberle 1 , Dillon T Flood 1 , Phil S Baran 1
Affiliation
|
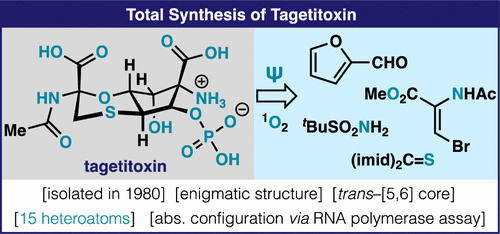
The intriguing structure of 1, a longstanding challenge in natural product synthesis, has been the subject of multiple revisions and has been confirmed through total synthesis. The route commences from a renewable furan starting material and features a number of unusual transformations (rearrangements, bromocyclization, P(V)-based phosphate installation) to arrive at the target in 15 steps. As the route was designed to enable access to both enantiomers, its absolute configuration could be assigned using a bioassay on (+)-1 and (-)-1.
中文翻译:

Tagetitoxin的全合成
1 的有趣结构是天然产物合成中长期存在的挑战,已成为多次修订的主题,并已通过全合成得到证实。该路线从可再生呋喃起始材料开始,并具有许多不寻常的转化(重排、溴环化、基于 P(V) 的磷酸盐安装),以通过 15 个步骤到达目标。由于该路线设计为能够获得两种对映异构体,因此可以使用 (+)-1 和 (-)-1 上的生物测定来确定其绝对构型。
更新日期:2020-07-20
中文翻译:

Tagetitoxin的全合成
1 的有趣结构是天然产物合成中长期存在的挑战,已成为多次修订的主题,并已通过全合成得到证实。该路线从可再生呋喃起始材料开始,并具有许多不寻常的转化(重排、溴环化、基于 P(V) 的磷酸盐安装),以通过 15 个步骤到达目标。由于该路线设计为能够获得两种对映异构体,因此可以使用 (+)-1 和 (-)-1 上的生物测定来确定其绝对构型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号