当前位置:
X-MOL 学术
›
Spectrochim. Acta B. At. Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition metal chromophores in glass beads of the classical and Hellenistic period: Bonding environment and colouring role
Spectrochimica Acta Part B: Atomic Spectroscopy ( IF 3.2 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.sab.2020.105928 Fani Pinakidou , Maria Katsikini , Eleni C. Paloura , Janos Osan , Mateusz Czyzycki , Alessandro Migliori , Eleni Palamara , Nikolaos Zacharias , Andreas Gemanos Karydas
Spectrochimica Acta Part B: Atomic Spectroscopy ( IF 3.2 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.sab.2020.105928 Fani Pinakidou , Maria Katsikini , Eleni C. Paloura , Janos Osan , Mateusz Czyzycki , Alessandro Migliori , Eleni Palamara , Nikolaos Zacharias , Andreas Gemanos Karydas
|
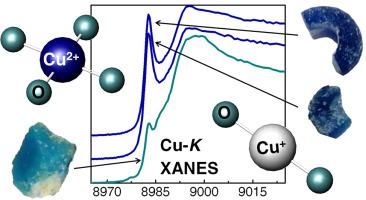
Abstract The delicate blue and green hue of ancient coloured beads from the Classical and Hellenistic period originate from the different transition metal (TM) ions added in the batch materials during glass production. The chemical composition in conjunction with the TM redox are fingerprints of the colour and vitrification process. We report on the structural and colouring/decolouring role of TM in blue and green-blue soda-lime beads from Thebes, central Greece dated back to the Classical and Hellenistic period using non-destructive X-Ray Absorption Fine Structure (XAFS) spectroscopies. Concerning the TMs redox and their consequent influence on colour, it is established that the major blue colourants are Co2+ and Cu2+, colourless Cu+ dominates in the blue beads, whereas chromophores Fe3+ and Cu2+ give rise to a green-blue hue. The Mn2+ content in the blue beads, whose occurrence is attributed to unintentional addition via the Co rich mineral colourant, counteracts the effect of the moderately higher content of Fe2+ in the raw materials. Based on the XAFS analysis results, we propose that the fractions of all TM under question (Mn, Fe, Co and Cu) were chosen and adjusted properly not only to control the desired colour, but also to ensure the stability of the glass matrix. Indeed, the integrity of the silica matrix should be further enhanced due to the presence of intermediate Fe oxides linking to Cu ions in either planar or linear geometry, while Mn and Co act as typical network formers.
中文翻译:

古典和希腊化时期玻璃珠中的过渡金属发色团:结合环境和着色作用
摘要 古典和希腊化时期的古代彩色珠子细腻的蓝绿色调源于玻璃生产过程中在配合料中添加的不同过渡金属(TM)离子。与 TM 氧化还原结合的化学成分是颜色和玻璃化过程的指纹。我们使用无损 X 射线吸收精细结构 (XAFS) 光谱报告了 TM 在希腊中部底比斯的蓝色和绿蓝色碱石灰珠中的结构和着色/脱色作用,其历史可以追溯到古典和希腊化时期。关于 TM 氧化还原及其对颜色的影响,已确定主要的蓝色着色剂是 Co2+ 和 Cu2+,无色 Cu+ 在蓝色珠粒中占主导地位,而发色团 Fe3+ 和 Cu2+ 产生绿蓝色调。蓝色珠粒中的 Mn2+ 含量(其发生归因于通过富含 Co 的矿物着色剂无意添加)抵消了原材料中 Fe2+ 含量适度较高的影响。根据 XAFS 分析结果,我们建议选择并适当调整所讨论的所有 TM 的分数(Mn、Fe、Co 和 Cu),不仅可以控制所需的颜色,还可以确保玻璃基质的稳定性。事实上,由于中间 Fe 氧化物以平面或线性几何形状与 Cu 离子连接,二氧化硅基质的完整性应该进一步增强,而 Mn 和 Co 作为典型的网络形成剂。抵消了原材料中 Fe2+ 含量较高的影响。根据 XAFS 分析结果,我们建议选择并适当调整所讨论的所有 TM 的分数(Mn、Fe、Co 和 Cu),不仅可以控制所需的颜色,还可以确保玻璃基质的稳定性。事实上,由于中间 Fe 氧化物以平面或线性几何形状与 Cu 离子连接,二氧化硅基质的完整性应该进一步增强,而 Mn 和 Co 作为典型的网络形成剂。抵消了原材料中 Fe2+ 含量较高的影响。根据 XAFS 分析结果,我们建议选择并适当调整所讨论的所有 TM 的分数(Mn、Fe、Co 和 Cu),不仅可以控制所需的颜色,还可以确保玻璃基质的稳定性。事实上,由于中间 Fe 氧化物以平面或线性几何形状与 Cu 离子连接,二氧化硅基质的完整性应该进一步增强,而 Mn 和 Co 作为典型的网络形成剂。
更新日期:2020-09-01
中文翻译:

古典和希腊化时期玻璃珠中的过渡金属发色团:结合环境和着色作用
摘要 古典和希腊化时期的古代彩色珠子细腻的蓝绿色调源于玻璃生产过程中在配合料中添加的不同过渡金属(TM)离子。与 TM 氧化还原结合的化学成分是颜色和玻璃化过程的指纹。我们使用无损 X 射线吸收精细结构 (XAFS) 光谱报告了 TM 在希腊中部底比斯的蓝色和绿蓝色碱石灰珠中的结构和着色/脱色作用,其历史可以追溯到古典和希腊化时期。关于 TM 氧化还原及其对颜色的影响,已确定主要的蓝色着色剂是 Co2+ 和 Cu2+,无色 Cu+ 在蓝色珠粒中占主导地位,而发色团 Fe3+ 和 Cu2+ 产生绿蓝色调。蓝色珠粒中的 Mn2+ 含量(其发生归因于通过富含 Co 的矿物着色剂无意添加)抵消了原材料中 Fe2+ 含量适度较高的影响。根据 XAFS 分析结果,我们建议选择并适当调整所讨论的所有 TM 的分数(Mn、Fe、Co 和 Cu),不仅可以控制所需的颜色,还可以确保玻璃基质的稳定性。事实上,由于中间 Fe 氧化物以平面或线性几何形状与 Cu 离子连接,二氧化硅基质的完整性应该进一步增强,而 Mn 和 Co 作为典型的网络形成剂。抵消了原材料中 Fe2+ 含量较高的影响。根据 XAFS 分析结果,我们建议选择并适当调整所讨论的所有 TM 的分数(Mn、Fe、Co 和 Cu),不仅可以控制所需的颜色,还可以确保玻璃基质的稳定性。事实上,由于中间 Fe 氧化物以平面或线性几何形状与 Cu 离子连接,二氧化硅基质的完整性应该进一步增强,而 Mn 和 Co 作为典型的网络形成剂。抵消了原材料中 Fe2+ 含量较高的影响。根据 XAFS 分析结果,我们建议选择并适当调整所讨论的所有 TM 的分数(Mn、Fe、Co 和 Cu),不仅可以控制所需的颜色,还可以确保玻璃基质的稳定性。事实上,由于中间 Fe 氧化物以平面或线性几何形状与 Cu 离子连接,二氧化硅基质的完整性应该进一步增强,而 Mn 和 Co 作为典型的网络形成剂。









































 京公网安备 11010802027423号
京公网安备 11010802027423号