当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural insights into the enhanced carbapenemase efficiency of OXA-655 compared to OXA-10.
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-08-08 , DOI: 10.1002/2211-5463.12935 Hanna-Kirsti S Leiros 1 , Ane Molden Thomassen 1 , Ørjan Samuelsen 2, 3 , Carl-Fredrik Flach 4, 5 , Stathis D Kotsakis 4, 5 , D G Joakim Larsson 4, 5
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-08-08 , DOI: 10.1002/2211-5463.12935 Hanna-Kirsti S Leiros 1 , Ane Molden Thomassen 1 , Ørjan Samuelsen 2, 3 , Carl-Fredrik Flach 4, 5 , Stathis D Kotsakis 4, 5 , D G Joakim Larsson 4, 5
Affiliation
|
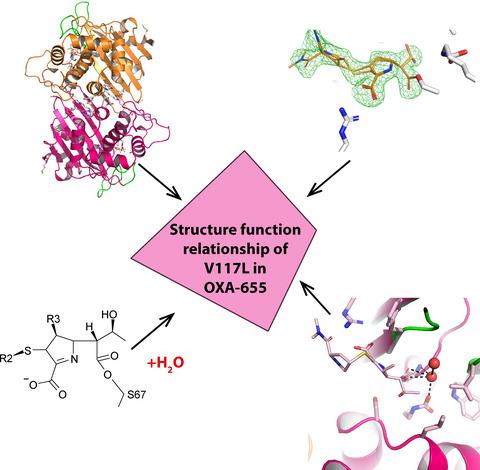
Carbapenemases are the main cause of carbapenem resistance in Gram‐negative bacteria. How β‐lactamases with weak carbapenemase activity, such as the OXA‐10‐type class D β‐lactamases, contribute to anti‐bacterial drug resistance is unclear. OXA‐655 is a T26M and V117L OXA‐10 variant, recently identified from hospital wastewater. Despite exhibiting stronger carbapenemase activity towards ertapenem (ETP) and meropenem (MEM) in Escherichia coli, OXA‐655 exhibits reduced activity towards oxyimino‐substituted β‐lactams like ceftazidime. Here, we have solved crystal structures of OXA‐10 in complex with imipenem (IPM) and ETP, and OXA‐655 in complex with MEM in order to unravel the structure–function relationship and the impact of residue 117 in enzyme catalysis. The new crystal structures show that L117 is situated at a critical position with enhanced Van der Waals interactions to L155 in the omega loop. This restricts the movements of L155 and could explain the reduced ability for OXA‐655 to bind a bulky oxyimino group. The V117L replacement in OXA‐655 makes the active site S67 and the carboxylated K70 more water exposed. This could affect the supply of new deacylation water molecules required for hydrolysis and possibly the carboxylation rate of K70. But most importantly, L117 leaves more space for binding of the hydroxyethyl group in carbapenems. In summary, the crystal structures highlight the importance of residue 117 in OXA‐10 variants for carbapenemase activity. This study also illustrates the impact of a single amino acid substitution on the substrate profile of OXA‐10 and the evolutionary potential of new OXA‐10 variants.
中文翻译:

与 OXA-10 相比,OXA-655 碳青霉烯酶效率增强的结构见解。
碳青霉烯酶是革兰氏阴性菌对碳青霉烯类耐药的主要原因。碳青霉烯酶活性较弱的 β-内酰胺酶,如 OXA-10 型 D 类β-内酰胺酶,如何导致抗菌药物耐药性尚不清楚。OXA-655 是 T26M 和 V117L OXA-10 变体,最近从医院废水中发现。尽管对大肠杆菌中的厄他培南 (ETP) 和美罗培南 (MEM) 表现出更强的碳青霉烯酶活性, OXA-655 对氧亚氨基取代的 β-内酰胺类药物(如头孢他啶)的活性降低。在这里,我们解决了 OXA-10 与亚胺培南 (IPM) 和 ETP 复合物的晶体结构,以及 OXA-655 与 MEM 复合物的晶体结构,以阐明结构-功能关系以及残基 117 在酶催化中的影响。新的晶体结构表明 L117 位于关键位置,在欧米茄环中与 L155 的范德华相互作用增强。这限制了 L155 的运动,并且可以解释 OXA-655 结合庞大的氧亚氨基的能力降低。OXA-655 中的 V117L 替代物使活性位点 S67 和羧化 K70 暴露于更多的水。这可能会影响水解所需的新脱酰水分子的供应,并可能影响 K70 的羧化速率。但最重要的是,L117 为碳青霉烯类中的羟乙基结合留出更多空间。总之,晶体结构突出了 OXA-10 变体中残基 117 对碳青霉烯酶活性的重要性。该研究还说明了单个氨基酸取代对 OXA-10 底物谱和新 OXA-10 变体的进化潜力的影响。
更新日期:2020-08-08
中文翻译:

与 OXA-10 相比,OXA-655 碳青霉烯酶效率增强的结构见解。
碳青霉烯酶是革兰氏阴性菌对碳青霉烯类耐药的主要原因。碳青霉烯酶活性较弱的 β-内酰胺酶,如 OXA-10 型 D 类β-内酰胺酶,如何导致抗菌药物耐药性尚不清楚。OXA-655 是 T26M 和 V117L OXA-10 变体,最近从医院废水中发现。尽管对大肠杆菌中的厄他培南 (ETP) 和美罗培南 (MEM) 表现出更强的碳青霉烯酶活性, OXA-655 对氧亚氨基取代的 β-内酰胺类药物(如头孢他啶)的活性降低。在这里,我们解决了 OXA-10 与亚胺培南 (IPM) 和 ETP 复合物的晶体结构,以及 OXA-655 与 MEM 复合物的晶体结构,以阐明结构-功能关系以及残基 117 在酶催化中的影响。新的晶体结构表明 L117 位于关键位置,在欧米茄环中与 L155 的范德华相互作用增强。这限制了 L155 的运动,并且可以解释 OXA-655 结合庞大的氧亚氨基的能力降低。OXA-655 中的 V117L 替代物使活性位点 S67 和羧化 K70 暴露于更多的水。这可能会影响水解所需的新脱酰水分子的供应,并可能影响 K70 的羧化速率。但最重要的是,L117 为碳青霉烯类中的羟乙基结合留出更多空间。总之,晶体结构突出了 OXA-10 变体中残基 117 对碳青霉烯酶活性的重要性。该研究还说明了单个氨基酸取代对 OXA-10 底物谱和新 OXA-10 变体的进化潜力的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号