Talanta ( IF 5.6 ) Pub Date : 2020-07-19 , DOI: 10.1016/j.talanta.2020.121406 Ioanna Dagla 1 , Anthony Tsarbopoulos 2 , Evagelos Gikas 3
|
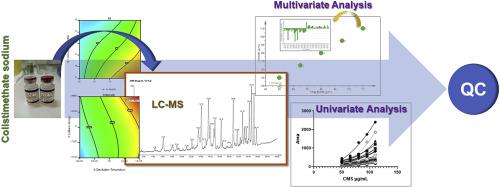
Colistimethate sodium (CMS) is a widely administrated old-generation prodrug for the treatment of the life-threatening infections caused by multi-resistant Gram-negative bacteria. Until now, the quality control procedure of the CMS commercial products is based on microbiological assays. The aim of the study is the development of a chemical analysis methodology based on liquid chromatography - mass spectrometry (LC-MS) that could be used for the quality control of CMS products. The careful optimization of the LC and QToF-MS parameters was deemed crucial, as CMS is known to be a very complex mixture. Thus, a two stage Design of Experiments (DoE) pipeline has been followed, aiming towards the separation of the mixture components. According to the DoE results, a baseline-resolved chromatogram revealing more than 20 compounds was achieved. The separation was performed using a Waters Acquity BEH C8 column employing gradient elution. The mobile phase consisted of aq. ammonium formate 0.005 M (pH 6) (solvent A) and methanol/acetonitrile 79/21 (v/v) (solvent B). A second optimization experiment for the MS signal was employed in order to achieve maximum sensitivity. The singly charged signals were monitored for the validation in the positive ion mode. The calibration curve range was 50–110 μg mL−1, corresponding to the 80–120% of the nominal CMS amount in the commercial products. Due to the complexity of the CMS chromatograms and the corresponding spectrum of each chromatographic peak, untargeted and targeted approaches were performed employing the MZmine software. Furthermore, apart from the classical univariate statistical analysis, partial least squares regression (PLS-R) model was also employed, as the variables were more than the observations. The developed methodology has been employed to analyze several batches and inconsistences have been discovered.
中文翻译:

实验设计指导了通过超高效液相色谱-高分辨率质谱法定量注射用大黄酮酸钠的多元校准。
二十烷基萘二甲磺酸钠(CMS)是一种广泛使用的老前药,用于治疗由多重耐药革兰氏阴性菌引起的威胁生命的感染。到目前为止,CMS商业产品的质量控制程序都是基于微生物测定的。该研究的目的是开发一种基于液相色谱-质谱(LC-MS)的化学分析方法,该方法可用于CMS产品的质量控制。LC和QToF-MS参数的仔细优化被认为至关重要,因为众所周知CMS是非常复杂的混合物。因此,遵循了两阶段的实验设计(DoE)流程,旨在分离混合物成分。根据DoE结果,获得了可分辨20多种化合物的基线分离色谱图。使用采用梯度洗脱的Waters Acquity BEH C8色谱柱进行分离。流动相由水溶液组成。甲酸铵0.005 M(pH 6)(溶剂A)和甲醇/乙腈79/21(v / v)(溶剂B)。为了获得最大灵敏度,对MS信号进行了第二次优化实验。监测单电荷信号在阳离子模式下的有效性。校准曲线范围是50–110μgmL 监测单电荷信号在阳离子模式下的有效性。校准曲线范围是50–110μgmL 监测单电荷信号在阳离子模式下的有效性。校准曲线范围是50–110μgmL-1,对应于商业产品中CMS名义上的80-120%。由于CMS色谱图的复杂性和每个色谱峰的相应光谱,因此使用MZmine软件执行了非靶向和靶向方法。此外,除了经典的单变量统计分析之外,还使用了偏最小二乘回归(PLS-R)模型,因为变量比观察值更多。所开发的方法已用于分析多个批次,并且发现了不一致之处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号