当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Human endothelial colony-forming cells in regenerative therapy: A systematic review of controlled preclinical animal studies.
STEM CELLS Translational Medicine ( IF 6 ) Pub Date : 2020-07-18 , DOI: 10.1002/sctm.20-0141 Gary Liao 1, 2 , Katina Zheng 2 , Risa Shorr 3 , David S Allan 1, 2
STEM CELLS Translational Medicine ( IF 6 ) Pub Date : 2020-07-18 , DOI: 10.1002/sctm.20-0141 Gary Liao 1, 2 , Katina Zheng 2 , Risa Shorr 3 , David S Allan 1, 2
Affiliation
|
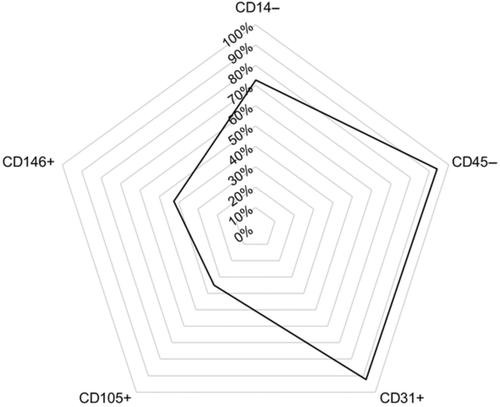
Endothelial colony‐forming cells (ECFCs) hold significant promise as candidates for regenerative therapy of vascular injury. Existing studies remain largely preclinical and exhibit marked design heterogeneity. A systematic review of controlled preclinical trials of human ECFCs is needed to guide future study design and to accelerate clinical translation. A systematic search of Medline and EMBASE on 1 April 2019 returned 3131 unique entries of which 66 fulfilled the inclusion criteria. Most studies used ECFCs derived from umbilical cord or adult peripheral blood. Studies used genetically modified immunodeficient mice (n = 52) and/or rats (n = 16). ECFC phenotypes were inconsistently characterized. While >90% of studies used CD31+ and CD45−, CD14− was demonstrated in 73% of studies, CD146+ in 42%, and CD10+ in 35%. Most disease models invoked ischemia. Peripheral vascular ischemia (n = 29), central nervous system ischemia (n = 14), connective tissue injury (n = 10), and cardiovascular ischemia and reperfusion injury (n = 7) were studied most commonly. Studies showed predominantly positive results; only 13 studies reported ≥1 outcome with null results, three reported only null results, and one reported harm. Quality assessment with SYRCLE revealed potential sources of bias in most studies. Preclinical ECFC studies are associated with benefit across several ischemic conditions in animal models, although combining results is limited by marked heterogeneity in study design. In particular, characterization of ECFCs varied and aspects of reporting introduced risk of bias in most studies. More studies with greater focus on standardized cell characterization and consistency of the disease model are needed.
中文翻译:

再生治疗中的人内皮集落形成细胞:对照临床前动物研究的系统评价。
内皮集落形成细胞(ECFCs)作为血管损伤再生治疗的候选者具有重要的前景。现有的研究仍然主要是临床前的,并表现出明显的设计异质性。需要对人类 ECFC 的受控临床前试验进行系统评价,以指导未来的研究设计并加速临床转化。2019 年 4 月 1 日对 Medline 和 EMBASE 的系统搜索返回了 3131 个独特的条目,其中 66 个符合纳入标准。大多数研究使用来自脐带或成人外周血的 ECFC。研究使用了转基因免疫缺陷小鼠(n = 52)和/或大鼠(n = 16)。ECFC 表型的特征不一致。虽然>90% 的研究使用了 CD31+ 和 CD45-,但 73% 的研究证实了 CD14-,42% 的研究证实了 CD146+,35% 的研究证实了 CD10+。大多数疾病模型调用缺血。外周血管缺血 (n = 29)、中枢神经系统缺血 (n = 14)、结缔组织损伤 (n = 10) 以及心血管缺血和再灌注损伤 (n = 7) 是最常被研究的。研究显示主要是积极的结果;只有 13 项研究报告了≥1 个结果且结果无效,3 项研究仅报告了无效结果,1 项报告了危害。SYRCLE 的质量评估揭示了大多数研究中潜在的偏倚来源。临床前 ECFC 研究与动物模型中几种缺血条件的益处相关,尽管合并结果受到研究设计的显着异质性的限制。特别是,ECFC 的特征各不相同,并且报告的各个方面在大多数研究中引入了偏倚风险。
更新日期:2020-07-18
中文翻译:

再生治疗中的人内皮集落形成细胞:对照临床前动物研究的系统评价。
内皮集落形成细胞(ECFCs)作为血管损伤再生治疗的候选者具有重要的前景。现有的研究仍然主要是临床前的,并表现出明显的设计异质性。需要对人类 ECFC 的受控临床前试验进行系统评价,以指导未来的研究设计并加速临床转化。2019 年 4 月 1 日对 Medline 和 EMBASE 的系统搜索返回了 3131 个独特的条目,其中 66 个符合纳入标准。大多数研究使用来自脐带或成人外周血的 ECFC。研究使用了转基因免疫缺陷小鼠(n = 52)和/或大鼠(n = 16)。ECFC 表型的特征不一致。虽然>90% 的研究使用了 CD31+ 和 CD45-,但 73% 的研究证实了 CD14-,42% 的研究证实了 CD146+,35% 的研究证实了 CD10+。大多数疾病模型调用缺血。外周血管缺血 (n = 29)、中枢神经系统缺血 (n = 14)、结缔组织损伤 (n = 10) 以及心血管缺血和再灌注损伤 (n = 7) 是最常被研究的。研究显示主要是积极的结果;只有 13 项研究报告了≥1 个结果且结果无效,3 项研究仅报告了无效结果,1 项报告了危害。SYRCLE 的质量评估揭示了大多数研究中潜在的偏倚来源。临床前 ECFC 研究与动物模型中几种缺血条件的益处相关,尽管合并结果受到研究设计的显着异质性的限制。特别是,ECFC 的特征各不相同,并且报告的各个方面在大多数研究中引入了偏倚风险。



























 京公网安备 11010802027423号
京公网安备 11010802027423号