当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
3,5-Dibromo-4-hydroxybenzaldehyde dissolved in aqueous solutions of ethanol, n-propanol, acetonitrile and N,N-dimethylformamide: Solubility modelling, solvent effect and preferential solvation investigation
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jct.2020.106252 Changfei Zhu , Renjie Xu , Hao Yin , Hongkun Zhao , Ali Farajtabar
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jct.2020.106252 Changfei Zhu , Renjie Xu , Hao Yin , Hongkun Zhao , Ali Farajtabar
|
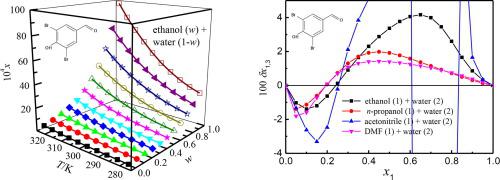
Abstract Equilibrium solubility of 3,5-dibromo-4-hydroxybenzaldehyde in four aqueous solutions of ethanol/n-propanol/acetonitrile/N,N-dimethylformamide (DMF) was determined at temperatures covering from 278.15 K to 323.15 K. All determinations were carried out by the well-known saturation shake-flask method under atmospheric pressure of 101.2 kPa. The maximum values of solubility data appeared in neat ethanol/n-propanol/acetonitrile/DMF for each solution. By Jouyban-Acree model, Apelblat-Jouyban-Acree model and van’t Hoff-Jouyban-Acree model, the 3,5-dibromo-4-hydroxybenzaldehyde solubility was well mathematically described obtaining RAD values smaller than 3.71% and RMSD values smaller than 2.27 × 10-4. The Kamlet and Taft linear solvation energy relationships were employed to examine the chief factors describing solvent effect. The cavity formation energy of solvents played a significant role on the solubility variation of 3,5-dibromo-4-hydroxybenzaldehyde in different aqueous solutions. The local mole fractions of ethanol/n-propanol/acetonitrile/DMF and water molecules around the 3,5-dibromo-4-hydroxybenzaldehyde molecule were quantitatively studied via the Inverse Kirkwood–Buff integrals way used to the solubility values. 3,5-Bibromo-4-hydroxybenzaldehyde was preferentially solvated by water in water-rich compositions for the ethanol/n-propanol/acetonitrile/DMF + water mixtures; while within co-solvent-rich and intermediate compositions, 3,5-dibromo-4-hydroxybenzaldehyde was preferentially solvated by ethanol/n-propanol/acetonitrile/DMF.
中文翻译:

溶解在乙醇、正丙醇、乙腈和 N,N-二甲基甲酰胺水溶液中的 3,5-二溴-4-羟基苯甲醛:溶解度建模、溶剂效应和优先溶剂化研究
摘要 测定了 3,5-二溴-4-羟基苯甲醛在乙醇/正丙醇/乙腈/N,N-二甲基甲酰胺 (DMF) 四种水溶液中的平衡溶解度,温度范围为 278.15 K 至 323.15 K。所有测定均进行在 101.2 kPa 的大气压下通过众所周知的饱和摇瓶法取出。对于每种溶液,溶解度数据的最大值出现在纯乙醇/正丙醇/乙腈/DMF 中。通过 Jouyban-Acree 模型、Apelblat-Jouyban-Acree 模型和 van't Hoff-Jouyban-Acree 模型,3,5-二溴-4-羟基苯甲醛的溶解度在数学上得到了很好的描述,获得的 RAD 值小于 3.71%,RMSD 值小于2.27 × 10-4。Kamlet 和 Taft 线性溶剂化能关系被用来检查描述溶剂效应的主要因素。溶剂的空穴形成能对3,5-二溴-4-羟基苯甲醛在不同水溶液中的溶解度变化有显着影响。通过用于溶解度值的逆柯克伍德-布夫积分方法,对乙醇/正丙醇/乙腈/DMF 和 3,5-二溴-4-羟基苯甲醛分子周围的水分子的局部摩尔分数进行了定量研究。在乙醇/正丙醇/乙腈/DMF+水混合物的富水组合物中,3,5-Bibromo-4-羟基苯甲醛优先被水溶剂化;而在富含共溶剂和中间体的组合物中,3,5-二溴-4-羟基苯甲醛优先被乙醇/正丙醇/乙腈/DMF 溶剂化。5-二溴-4-羟基苯甲醛在不同的水溶液中。通过用于溶解度值的逆柯克伍德-布夫积分方法,对乙醇/正丙醇/乙腈/DMF 和 3,5-二溴-4-羟基苯甲醛分子周围的水分子的局部摩尔分数进行了定量研究。在乙醇/正丙醇/乙腈/DMF+水混合物的富水组合物中,3,5-Bibromo-4-羟基苯甲醛优先被水溶剂化;而在富含共溶剂和中间体的组合物中,3,5-二溴-4-羟基苯甲醛优先被乙醇/正丙醇/乙腈/DMF 溶剂化。5-二溴-4-羟基苯甲醛在不同的水溶液中。通过用于溶解度值的逆柯克伍德-布夫积分方法,对乙醇/正丙醇/乙腈/DMF 和 3,5-二溴-4-羟基苯甲醛分子周围的水分子的局部摩尔分数进行了定量研究。在乙醇/正丙醇/乙腈/DMF+水混合物的富水组合物中,3,5-Bibromo-4-羟基苯甲醛优先被水溶剂化;而在富含共溶剂和中间体的组合物中,3,5-二溴-4-羟基苯甲醛优先被乙醇/正丙醇/乙腈/DMF 溶剂化。通过用于溶解度值的逆柯克伍德-布夫积分方法对 5-二溴-4-羟基苯甲醛分子进行了定量研究。在乙醇/正丙醇/乙腈/DMF+水混合物的富水组合物中,3,5-Bibromo-4-羟基苯甲醛优先被水溶剂化;而在富含共溶剂和中间体的组合物中,3,5-二溴-4-羟基苯甲醛优先被乙醇/正丙醇/乙腈/DMF 溶剂化。通过用于溶解度值的逆柯克伍德-布夫积分方法对 5-二溴-4-羟基苯甲醛分子进行了定量研究。在乙醇/正丙醇/乙腈/DMF+水混合物的富水组合物中,3,5-Bibromo-4-羟基苯甲醛优先被水溶剂化;而在富含共溶剂和中间体的组合物中,3,5-二溴-4-羟基苯甲醛优先被乙醇/正丙醇/乙腈/DMF 溶剂化。
更新日期:2020-12-01
中文翻译:

溶解在乙醇、正丙醇、乙腈和 N,N-二甲基甲酰胺水溶液中的 3,5-二溴-4-羟基苯甲醛:溶解度建模、溶剂效应和优先溶剂化研究
摘要 测定了 3,5-二溴-4-羟基苯甲醛在乙醇/正丙醇/乙腈/N,N-二甲基甲酰胺 (DMF) 四种水溶液中的平衡溶解度,温度范围为 278.15 K 至 323.15 K。所有测定均进行在 101.2 kPa 的大气压下通过众所周知的饱和摇瓶法取出。对于每种溶液,溶解度数据的最大值出现在纯乙醇/正丙醇/乙腈/DMF 中。通过 Jouyban-Acree 模型、Apelblat-Jouyban-Acree 模型和 van't Hoff-Jouyban-Acree 模型,3,5-二溴-4-羟基苯甲醛的溶解度在数学上得到了很好的描述,获得的 RAD 值小于 3.71%,RMSD 值小于2.27 × 10-4。Kamlet 和 Taft 线性溶剂化能关系被用来检查描述溶剂效应的主要因素。溶剂的空穴形成能对3,5-二溴-4-羟基苯甲醛在不同水溶液中的溶解度变化有显着影响。通过用于溶解度值的逆柯克伍德-布夫积分方法,对乙醇/正丙醇/乙腈/DMF 和 3,5-二溴-4-羟基苯甲醛分子周围的水分子的局部摩尔分数进行了定量研究。在乙醇/正丙醇/乙腈/DMF+水混合物的富水组合物中,3,5-Bibromo-4-羟基苯甲醛优先被水溶剂化;而在富含共溶剂和中间体的组合物中,3,5-二溴-4-羟基苯甲醛优先被乙醇/正丙醇/乙腈/DMF 溶剂化。5-二溴-4-羟基苯甲醛在不同的水溶液中。通过用于溶解度值的逆柯克伍德-布夫积分方法,对乙醇/正丙醇/乙腈/DMF 和 3,5-二溴-4-羟基苯甲醛分子周围的水分子的局部摩尔分数进行了定量研究。在乙醇/正丙醇/乙腈/DMF+水混合物的富水组合物中,3,5-Bibromo-4-羟基苯甲醛优先被水溶剂化;而在富含共溶剂和中间体的组合物中,3,5-二溴-4-羟基苯甲醛优先被乙醇/正丙醇/乙腈/DMF 溶剂化。5-二溴-4-羟基苯甲醛在不同的水溶液中。通过用于溶解度值的逆柯克伍德-布夫积分方法,对乙醇/正丙醇/乙腈/DMF 和 3,5-二溴-4-羟基苯甲醛分子周围的水分子的局部摩尔分数进行了定量研究。在乙醇/正丙醇/乙腈/DMF+水混合物的富水组合物中,3,5-Bibromo-4-羟基苯甲醛优先被水溶剂化;而在富含共溶剂和中间体的组合物中,3,5-二溴-4-羟基苯甲醛优先被乙醇/正丙醇/乙腈/DMF 溶剂化。通过用于溶解度值的逆柯克伍德-布夫积分方法对 5-二溴-4-羟基苯甲醛分子进行了定量研究。在乙醇/正丙醇/乙腈/DMF+水混合物的富水组合物中,3,5-Bibromo-4-羟基苯甲醛优先被水溶剂化;而在富含共溶剂和中间体的组合物中,3,5-二溴-4-羟基苯甲醛优先被乙醇/正丙醇/乙腈/DMF 溶剂化。通过用于溶解度值的逆柯克伍德-布夫积分方法对 5-二溴-4-羟基苯甲醛分子进行了定量研究。在乙醇/正丙醇/乙腈/DMF+水混合物的富水组合物中,3,5-Bibromo-4-羟基苯甲醛优先被水溶剂化;而在富含共溶剂和中间体的组合物中,3,5-二溴-4-羟基苯甲醛优先被乙醇/正丙醇/乙腈/DMF 溶剂化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号