当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of C‒H bond in the antioxidant activities of rooperol and its derivatives: A DFT study
Phytochemistry ( IF 3.2 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.phytochem.2020.112454 Yan-Zhen Zheng 1 , Zhong-Min Fu 1 , Geng Deng 2 , Rui Guo 1 , Da-Fu Chen 1
Phytochemistry ( IF 3.2 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.phytochem.2020.112454 Yan-Zhen Zheng 1 , Zhong-Min Fu 1 , Geng Deng 2 , Rui Guo 1 , Da-Fu Chen 1
Affiliation
|
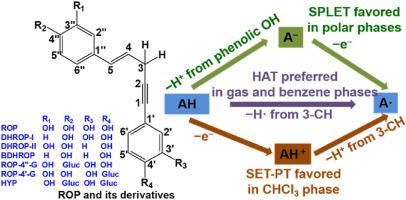
Rooperol and its derivatives, derived from the Hypoxis rooperi plant, are polyphenolic and norlignan compounds with excellent antioxidant activities. The reaction enthalpies for the free-radical scavenging by rooperol and its six derivatives were studied using density functional theory. We found that the C-H groups played a significant role in the antioxidant activities in non-polar phases. In the gas and benzene phases, rooperol and its derivatives preferentially underwent the free-radical scavenging process via the 3‒CH group by following the hydrogen atom transfer (HAT) mechanism. In polar phases, the sequential proton loss electron transfer (SPLET) was the most preferred mechanism, and the phenolic O‒H groups played a significant role. Additionally, we found that when the hydrogen atom in the OH group was replaced by a glucose moiety, the antioxidant activity of the adjacent OH group was reduced. ROP, DHROP-I, DHROP-II, ROP-4″-G and ROP-4'G have catechol moiety, they may proceed double step-wise mechanisms to trap free radicals. In the gas and benzene phases, the preferable mechanism is dHAT. In water phase, it is SPLHAT.
中文翻译:

C-H 键在鲁柏醇及其衍生物抗氧化活性中的作用:DFT 研究
Rooperol 及其衍生物来源于 Hypoxis rooperi 植物,是具有优异抗氧化活性的多酚和降木脂素化合物。使用密度泛函理论研究了rooperol及其六种衍生物清除自由基的反应焓。我们发现 CH 基团在非极性相中的抗氧化活性中起重要作用。在气相和苯相中,Rooperol 及其衍生物通过遵循氢原子转移 (HAT) 机制通过 3-CH 基团优先进行自由基清除过程。在极性相中,顺序质子丢失电子转移(SPLET)是最优选的机制,酚类 O-H 基团发挥了重要作用。此外,我们发现当 OH 基团中的氢原子被葡萄糖部分取代时,相邻OH基团的抗氧化活性降低。ROP、DHROP-I、DHROP-II、ROP-4″-G 和 ROP-4'G 具有邻苯二酚部分,它们可以进行双重逐步机制来捕获自由基。在气相和苯相中,优选的机理是 dHAT。在水相中,它是 SPLHAT。
更新日期:2020-10-01
中文翻译:

C-H 键在鲁柏醇及其衍生物抗氧化活性中的作用:DFT 研究
Rooperol 及其衍生物来源于 Hypoxis rooperi 植物,是具有优异抗氧化活性的多酚和降木脂素化合物。使用密度泛函理论研究了rooperol及其六种衍生物清除自由基的反应焓。我们发现 CH 基团在非极性相中的抗氧化活性中起重要作用。在气相和苯相中,Rooperol 及其衍生物通过遵循氢原子转移 (HAT) 机制通过 3-CH 基团优先进行自由基清除过程。在极性相中,顺序质子丢失电子转移(SPLET)是最优选的机制,酚类 O-H 基团发挥了重要作用。此外,我们发现当 OH 基团中的氢原子被葡萄糖部分取代时,相邻OH基团的抗氧化活性降低。ROP、DHROP-I、DHROP-II、ROP-4″-G 和 ROP-4'G 具有邻苯二酚部分,它们可以进行双重逐步机制来捕获自由基。在气相和苯相中,优选的机理是 dHAT。在水相中,它是 SPLHAT。











































 京公网安备 11010802027423号
京公网安备 11010802027423号