当前位置:
X-MOL 学术
›
Nano Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A metal-free nanozyme-activated prodrug strategy for targeted tumor catalytic therapy
Nano Today ( IF 13.2 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.nantod.2020.100935 Qian Liang , Juqun Xi , Xuejiao J. Gao , Ruofei Zhang , Yili Yang , Xingfa Gao , Xiyun Yan , Lizeng Gao , Kelong Fan
Nano Today ( IF 13.2 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.nantod.2020.100935 Qian Liang , Juqun Xi , Xuejiao J. Gao , Ruofei Zhang , Yili Yang , Xingfa Gao , Xiyun Yan , Lizeng Gao , Kelong Fan
|
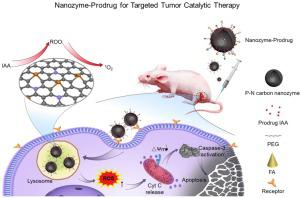
Abstract Enzyme-mediated activation of prodrug in tumor cells is an attractive strategy for targeted cancer therapy. Of note, natural peroxidase and indole-3-acetic acid (IAA) have been shown as a promising enzyme-prodrug combination. However, insufficient endogenous peroxidase activity and the difficulty in selectively expressing exogenous peroxidase in tumor cells have severely limited its antitumor efficiency. The discovery of nanomaterials with peroxidase-like activity (nanozyme), particularly the metal-free nanozymes that possesses high stability and good biocompatibility, bring a new opportunity to overcome these challenges. Here, under the guidance of theoretical calculations, we developed a metal-free phosphorous and nitrogen dual-doped porous hollow carbon sphere nanozyme (PNCNzyme) with robust peroxidase-like activity in acidic environment. Based on this metal-free nanozyme, we developed a nanozyme-IAA activation strategy that activated IAA to produce abundant ROS and trigger tumor cell apoptosis. We also introduced folate (FA) onto the nanozyme to enhance its tumor targeting and endocytosis efficacy by tumor cells. Upon administrated to mice, the FA-PNCNzymes@IAA effectively accumulated in the xenografts derived from cervical cancer cells, catalyzed the production of ROS, and induced apoptosis. Taken together, these data demonstrate that the nanozyme-IAA combination is an effective enzyme-prodrug activation strategy for tumor targeted catalytic therapy.
中文翻译:

用于靶向肿瘤催化治疗的无金属纳米酶激活前药策略
摘要 肿瘤细胞中酶介导的前药激活是靶向癌症治疗的一种有吸引力的策略。值得注意的是,天然过氧化物酶和吲哚-3-乙酸 (IAA) 已被证明是一种很有前景的酶-前药组合。然而,内源性过氧化物酶活性不足,外源性过氧化物酶在肿瘤细胞中难以选择性表达,严重限制了其抗肿瘤效率。具有类过氧化物酶活性的纳米材料(纳米酶)的发现,特别是具有高稳定性和良好生物相容性的无金属纳米酶,为克服这些挑战带来了新的机遇。在此,在理论计算的指导下,我们开发了一种无金属磷氮双掺杂多孔空心碳球纳米酶(PNCNzyme),在酸性环境中具有强大的类过氧化物酶活性。基于这种不含金属的纳米酶,我们开发了一种纳米酶-IAA 激活策略,该策略激活 IAA 以产生丰富的 ROS 并触发肿瘤细胞凋亡。我们还将叶酸 (FA) 引入纳米酶以增强其肿瘤靶向和肿瘤细胞的内吞作用。对小鼠给药后,FA-PNCNzymes@IAA 有效地积聚在宫颈癌细胞的异种移植物中,催化活性氧的产生,并诱导细胞凋亡。总之,这些数据表明纳米酶-IAA 组合是用于肿瘤靶向催化治疗的有效酶-前药激活策略。我们开发了一种纳米酶-IAA 激活策略,可激活 IAA 以产生丰富的 ROS 并触发肿瘤细胞凋亡。我们还将叶酸 (FA) 引入纳米酶以增强其肿瘤靶向和肿瘤细胞的内吞作用。对小鼠给药后,FA-PNCNzymes@IAA 有效地积聚在宫颈癌细胞的异种移植物中,催化活性氧的产生,并诱导细胞凋亡。总之,这些数据表明纳米酶-IAA 组合是用于肿瘤靶向催化治疗的有效酶-前药激活策略。我们开发了一种纳米酶-IAA 激活策略,可激活 IAA 以产生丰富的 ROS 并触发肿瘤细胞凋亡。我们还将叶酸 (FA) 引入纳米酶以增强其肿瘤靶向和肿瘤细胞的内吞作用。对小鼠给药后,FA-PNCNzymes@IAA 有效地积聚在宫颈癌细胞的异种移植物中,催化活性氧的产生,并诱导细胞凋亡。总之,这些数据表明纳米酶-IAA 组合是一种有效的酶-前药激活策略,用于肿瘤靶向催化治疗。FA-PNCNzymes@IAA在宫颈癌细胞异种移植物中有效积累,催化ROS的产生,并诱导细胞凋亡。总之,这些数据表明纳米酶-IAA 组合是用于肿瘤靶向催化治疗的有效酶-前药激活策略。FA-PNCNzymes@IAA在宫颈癌细胞异种移植物中有效积累,催化ROS的产生,并诱导细胞凋亡。总之,这些数据表明纳米酶-IAA 组合是用于肿瘤靶向催化治疗的有效酶-前药激活策略。
更新日期:2020-12-01
中文翻译:

用于靶向肿瘤催化治疗的无金属纳米酶激活前药策略
摘要 肿瘤细胞中酶介导的前药激活是靶向癌症治疗的一种有吸引力的策略。值得注意的是,天然过氧化物酶和吲哚-3-乙酸 (IAA) 已被证明是一种很有前景的酶-前药组合。然而,内源性过氧化物酶活性不足,外源性过氧化物酶在肿瘤细胞中难以选择性表达,严重限制了其抗肿瘤效率。具有类过氧化物酶活性的纳米材料(纳米酶)的发现,特别是具有高稳定性和良好生物相容性的无金属纳米酶,为克服这些挑战带来了新的机遇。在此,在理论计算的指导下,我们开发了一种无金属磷氮双掺杂多孔空心碳球纳米酶(PNCNzyme),在酸性环境中具有强大的类过氧化物酶活性。基于这种不含金属的纳米酶,我们开发了一种纳米酶-IAA 激活策略,该策略激活 IAA 以产生丰富的 ROS 并触发肿瘤细胞凋亡。我们还将叶酸 (FA) 引入纳米酶以增强其肿瘤靶向和肿瘤细胞的内吞作用。对小鼠给药后,FA-PNCNzymes@IAA 有效地积聚在宫颈癌细胞的异种移植物中,催化活性氧的产生,并诱导细胞凋亡。总之,这些数据表明纳米酶-IAA 组合是用于肿瘤靶向催化治疗的有效酶-前药激活策略。我们开发了一种纳米酶-IAA 激活策略,可激活 IAA 以产生丰富的 ROS 并触发肿瘤细胞凋亡。我们还将叶酸 (FA) 引入纳米酶以增强其肿瘤靶向和肿瘤细胞的内吞作用。对小鼠给药后,FA-PNCNzymes@IAA 有效地积聚在宫颈癌细胞的异种移植物中,催化活性氧的产生,并诱导细胞凋亡。总之,这些数据表明纳米酶-IAA 组合是用于肿瘤靶向催化治疗的有效酶-前药激活策略。我们开发了一种纳米酶-IAA 激活策略,可激活 IAA 以产生丰富的 ROS 并触发肿瘤细胞凋亡。我们还将叶酸 (FA) 引入纳米酶以增强其肿瘤靶向和肿瘤细胞的内吞作用。对小鼠给药后,FA-PNCNzymes@IAA 有效地积聚在宫颈癌细胞的异种移植物中,催化活性氧的产生,并诱导细胞凋亡。总之,这些数据表明纳米酶-IAA 组合是一种有效的酶-前药激活策略,用于肿瘤靶向催化治疗。FA-PNCNzymes@IAA在宫颈癌细胞异种移植物中有效积累,催化ROS的产生,并诱导细胞凋亡。总之,这些数据表明纳米酶-IAA 组合是用于肿瘤靶向催化治疗的有效酶-前药激活策略。FA-PNCNzymes@IAA在宫颈癌细胞异种移植物中有效积累,催化ROS的产生,并诱导细胞凋亡。总之,这些数据表明纳米酶-IAA 组合是用于肿瘤靶向催化治疗的有效酶-前药激活策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号