Cell Host & Microbe ( IF 20.6 ) Pub Date : 2020-07-18 , DOI: 10.1016/j.chom.2020.07.005 Nan Wang 1 , Yan Zhan 2 , Linyu Zhu 3 , Zhibing Hou 4 , Feng Liu 5 , Pinhong Song 5 , Feng Qiu 5 , Xiaolin Wang 6 , Xiafei Zou 6 , Deyun Wan 7 , Xiaosong Qian 7 , Shanshan Wang 8 , Yabi Guo 8 , Hao Yu 8 , Miao Cui 9 , Gangling Tong 10 , Yunsheng Xu 11 , Zhihua Zheng 12 , Yingying Lu 13 , Peng Hong 14
|
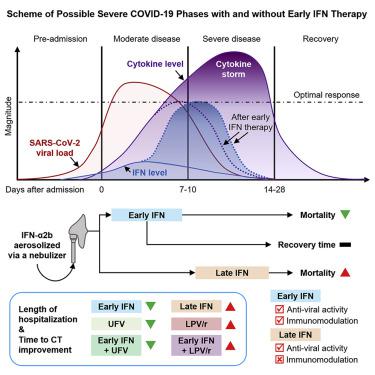
Interferons (IFNs) are widely used in treating coronavirus disease 2019 (COVID-19) patients. However, a recent report of ACE2, the host factor mediating SARS-Cov-2 infection, identifying it as interferon-stimulated raised considerable safety concern. To examine the association between the use and timing of IFN-α2b and clinical outcomes, we analyzed in a retrospective multicenter cohort study of 446 COVID-19 patients in Hubei, China. Regression models estimated that early administration (≤5 days after admission) of IFN-α2b was associated with reduced in-hospital mortality in comparison with no admission of IFN-α2b, whereas late administration of IFN-α2b was associated with increased mortality. Among survivors, early IFN-α2b was not associated with hospital discharge or computed tomography (CT) scan improvement, whereas late IFN-α2b was associated with delayed recovery. Additionally, early IFN-α2b and umifenovir alone or together were associated with reduced mortality and accelerated recovery in comparison with treatment with lopinavir/ritonavir (LPV/r) alone. We concluded that administration of IFN-α2b during the early stage of COVID-19 could induce favorable clinical responses.











































 京公网安备 11010802027423号
京公网安备 11010802027423号