当前位置:
X-MOL 学术
›
Cell Calcium
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Human TRPA1 is an inherently mechanosensitive bilayer-gated ion channel.
Cell Calcium ( IF 4 ) Pub Date : 2020-07-18 , DOI: 10.1016/j.ceca.2020.102255 Lavanya Moparthi 1 , Peter M Zygmunt 2
中文翻译:

人类 TRPA1 是一种固有的机械敏感双层门控离子通道。
更新日期:2020-07-24
Cell Calcium ( IF 4 ) Pub Date : 2020-07-18 , DOI: 10.1016/j.ceca.2020.102255 Lavanya Moparthi 1 , Peter M Zygmunt 2
Affiliation
|
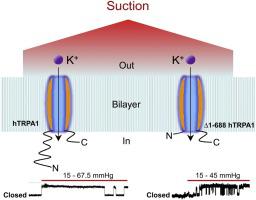
The role of mammalian Transient Receptor Potential Ankyrin 1 (TRPA1) as a mechanosensor is controversial. Here, we report that purified human TRPA1 (hTRPA1) with and without its N-terminal ankyrin repeat domain responded with pressure-dependent single-channel current activity when reconstituted into artificial lipid bilayers. The hTRPA1 activity was abolished by the thiol reducing agent TCEP. Thus, depending on its redox state, hTRPA1 is an inherent mechanosensitive ion channel gated by force-from-lipids.
中文翻译:

人类 TRPA1 是一种固有的机械敏感双层门控离子通道。
哺乳动物瞬时受体电位锚蛋白 1 (TRPA1) 作为机械传感器的作用是有争议的。在这里,我们报告纯化的人类 TRPA1 (hTRPA1) 有和没有其 N 端锚蛋白重复结构域,当重组为人工脂质双层时,其响应于压力依赖性单通道电流活动。hTRPA1 活性被硫醇还原剂 TCEP 消除。因此,根据其氧化还原状态,hTRPA1 是一种由脂质力门控的固有机械敏感离子通道。



























 京公网安备 11010802027423号
京公网安备 11010802027423号