Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2020-07-18 , DOI: 10.1016/j.apsb.2020.07.006 Hobin Yang 1 , Quoc-Viet Le 2 , Gayong Shim 2 , Yu-Kyoung Oh 2 , Young Kee Shin 1, 3, 4
|
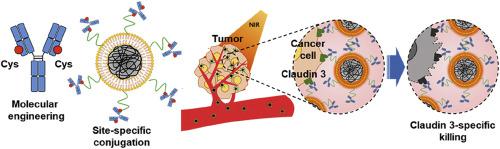
Conjugation of antibodies to nanoparticles allows specific cancer targeting, but conventional conjugation methods generate heterogeneous conjugations that cannot guarantee the optimal orientation and functionality of the conjugated antibody. Here, a molecular engineering technique was used for site-specific conjugation of antibodies to nanoparticles. We designed an anti-claudin 3 (CLDN3) antibody containing a single cysteine residue, h4G3cys, then linked it to the maleimide group of lipid polydopamine hybrid nanoparticles (LPNs). Because of their negatively charged lipid coating, LPNs showed high colloidal stability and provided a functional surface for site-specific conjugation of h4G3cys. The activity of h4G3cys was tested by measuring the binding of h4G3cys-conjugated LPNs (C-LPNs) to CLDN3-positive tumor cells and assessing its subsequent photothermal effects. C-LPNsspecifically recognized CLDN3-overexpressing T47D breast cancer cells but not CLDN3-negative Hs578T breast cancer cells. High binding of C-LPNs to CLDN3-overexpressing T47D cells resulted in significantly higher temperature generation upon NIR irradiation and potent anticancer photothermal efficacy. Consistent with this, intravenous injection of C-LPNsin a T47D xenograft mouse model followed by NIR irradiation caused remarkable tumor ablation compared with other treatments through high temperature increases. Our results establish an accurate antibody-linking method and demonstrate the possibility of developing therapeutics using antibody-guided nanoparticles.
中文翻译:

用于与脂质聚多巴胺杂化纳米粒子位点特异性缀合的抗体的分子工程
抗体与纳米颗粒的缀合可以实现特定的癌症靶向,但传统的缀合方法会产生异质缀合,无法保证缀合抗体的最佳方向和功能。在这里,分子工程技术用于抗体与纳米颗粒的位点特异性缀合。我们设计了一种含有单个半胱氨酸残基 h4G3cys 的抗密蛋白 3 (CLDN3) 抗体,然后将其与脂质聚多巴胺杂化纳米颗粒 (LPN) 的马来酰亚胺基团连接。由于其带负电荷的脂质涂层,LPN 显示出高胶体稳定性,并为 h4G3cys 的位点特异性缀合提供了功能表面。通过测量 h4G3cys 缀合的 LPN(C-LPN)与 CLDN3 阳性肿瘤细胞的结合并评估其随后的光热效应来测试 h4G3cys 的活性。 C-LPN 特异性识别 CLDN3 过表达的 T47D 乳腺癌细胞,但不识别 CLDN3 阴性的 Hs578T 乳腺癌细胞。 C-LPN 与 CLDN3 过表达的 T47D 细胞的高度结合导致近红外照射时产生显着更高的温度,并具有有效的抗癌光热功效。与此相一致的是,与通过高温升高的其他治疗相比,在 T47D 异种移植小鼠模型中静脉注射 C-LPNs,然后进行近红外照射,可引起显着的肿瘤消融。我们的结果建立了一种准确的抗体连接方法,并证明了使用抗体引导的纳米颗粒开发治疗方法的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号