当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic activity and mechanism of fluorinated MgO film supported on 3D nickel mesh for ozonation of gaseous toluene
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-07-17 , DOI: 10.1039/d0en00475h Jinzhu Zhu 1, 2, 3, 4, 5 , Jingxiang Sun 1, 2, 3, 4, 5 , Shuanghong Tian 1, 2, 3, 4, 5 , Juan Yang 1, 2, 3, 4, 5 , Jinxi Feng 1, 2, 3, 4, 5 , Ya Xiong 1, 2, 3, 4, 5
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-07-17 , DOI: 10.1039/d0en00475h Jinzhu Zhu 1, 2, 3, 4, 5 , Jingxiang Sun 1, 2, 3, 4, 5 , Shuanghong Tian 1, 2, 3, 4, 5 , Juan Yang 1, 2, 3, 4, 5 , Jinxi Feng 1, 2, 3, 4, 5 , Ya Xiong 1, 2, 3, 4, 5
Affiliation
|
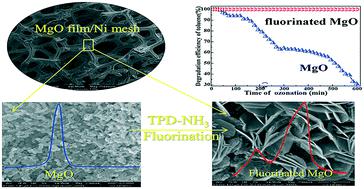
A nano-MgO film supported on 3D nickel mesh was prepared via an electrodeposition–thermolysis process. The film possessed high catalytic activity for the ozonation of toluene, but suffered from deactivation. Interestingly, after fluorination, its breakthrough time was 20 times longer (i.e., from 30 to 600 min) under 50% relative humidity. This significant enhancement was attributed to the formation of a fluorinated MgO film, Mg(OH)F, as confirmed using XRD, XPS, SEM, etc. Mg(OH)F possessed two typical acidic sites (Brønsted and Lewis sites), and they could co-catalyze O3 to generate more highly active ˙OH and O radicals. In particular, its Brønsted acidity could considerably decrease the accumulation of acidic intermediates on the film, suppressing its deactivation. Fluorinated MgO, Mg(OH)F, could be applied as a multifunctional ozonation catalyst for the degradation of other refractory pollutants.
中文翻译:

负载在3D镍网上的氟化MgO膜的催化活性及其对臭氧氧化的机理
通过电沉积-热解工艺制备了负载在3D镍网上的纳米MgO膜。该膜对甲苯的臭氧化具有高催化活性,但会失活。有趣的是,氟化后,在50%相对湿度下,其穿透时间延长了20倍(即从30到600分钟)。如XRD,XPS,SEM等所证实,这种显着增强归因于氟化MgO膜Mg(OH)F的形成。Mg(OH)F具有两个典型的酸性位点(Brønsted和Lewis位点),它们可以共催化O 3产生更高活性的˙OH和O自由基。特别是,其布朗斯台德酸度可以大大减少酸性中间体在薄膜上的积累,从而抑制其失活。氟化MgO,Mg(OH)F可用作多功能臭氧化催化剂,用于降解其他难处理污染物。
更新日期:2020-09-18
中文翻译:

负载在3D镍网上的氟化MgO膜的催化活性及其对臭氧氧化的机理
通过电沉积-热解工艺制备了负载在3D镍网上的纳米MgO膜。该膜对甲苯的臭氧化具有高催化活性,但会失活。有趣的是,氟化后,在50%相对湿度下,其穿透时间延长了20倍(即从30到600分钟)。如XRD,XPS,SEM等所证实,这种显着增强归因于氟化MgO膜Mg(OH)F的形成。Mg(OH)F具有两个典型的酸性位点(Brønsted和Lewis位点),它们可以共催化O 3产生更高活性的˙OH和O自由基。特别是,其布朗斯台德酸度可以大大减少酸性中间体在薄膜上的积累,从而抑制其失活。氟化MgO,Mg(OH)F可用作多功能臭氧化催化剂,用于降解其他难处理污染物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号