Current Proteomics ( IF 0.5 ) Pub Date : 2020-09-30 , DOI: 10.2174/1570164617666191017140456 Peng Liu 1 , Libo Hou 2 , Min Liu 2 , Xuechuan Xu 2 , Qi Gao 2 , Jiewen Deng 1 , Shasha Xiang 1 , Qian Cao 1 , Min Zhou 1 , Quanjie Yang 1 , Wen Wang 2 , Wei Gu 2 , Qingguo Meng 2
|
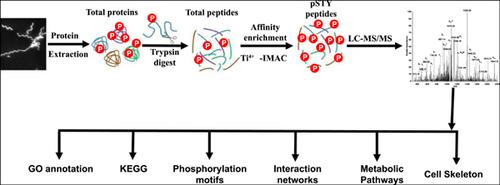
Background: Post-Translational Modifications (PTMs), such as phosphorylation, are an essential regulatory mechanism of protein function and are associated with a range of biological processes beyond the genome and transcriptome. Spiroplasma eriocheiris, a wall-less helical bacterium, is one of the smallest self-replicating bacteria, and is a novel pathogen of freshwater crustaceans.
Methods: Protein phosphorylation in S. eriocheiris was systematically investigated by iTRAQ analyzed by LC-MS/MS to study the physiological characteristics and regulatory mechanisms of this bacteria. Data are available via ProteomeXchange with identifier PXD015055.
Results: We identified 465 phosphorylation sites in 246 proteins involved in a broad spectrum of fundamental biological processes ranging from the regulation of metabolic pathways to protein synthesis. Notably, most proteins involved in glycolysis and all proteins in the arginine deiminase system were phosphorylated. Cytoskeleton proteins (Fibril, Mrebs and EF-Tu) were also phosphorylated suggesting that phosphorylation may play a crucial role in the formation of the cell skeleton. The analysis identified a number of highly conserved proteins and phosphorylation sites that predominantly participate in glucose metabolism and protein synthesis. Crosstalk analysis with protein-protein interaction networks in relation to phosphorylated proteins and acetylated proteins found that the two PTMs are involved in a number of crucial physiological processes in S. eriocheiris. Comparison of the relative positions of acetylation versus phosphorylation revealed that the two modifications are often located in close proximity to the same protein.
Conclusion: The results indicate a previously unreported role of phosphorylation in defining the functional state of Spiroplasma.
中文翻译:

的磷酸化蛋白质组学分析螺原体eriocheiris和串扰Acetylome揭示翻译后修饰的代谢中的作用
背景:翻译后修饰(PTM)(例如磷酸化)是蛋白质功能的基本调节机制,并且与基因组和转录组以外的一系列生物过程有关。螺旋藻eriocheiris是一种无壁螺旋细菌,是最小的自我复制细菌之一,是淡水甲壳类动物的新型病原体。
方法:采用LC-MS / MS技术,通过iTRAQ系统分析er.cheiosiris中蛋白质的磷酸化,研究该细菌的生理特性和调控机制。数据可通过ProteomeXchange获得,其标识符为PXD015055。
结果:我们在246种蛋白质中鉴定出465个磷酸化位点,这些蛋白质参与了从代谢途径的调控到蛋白质合成的广泛基础生物学过程。值得注意的是,大多数参与糖酵解的蛋白质和精氨酸脱亚氨酶系统中的所有蛋白质都被磷酸化了。细胞骨架蛋白(纤维蛋白,Mreebs和EF-Tu)也被磷酸化,表明磷酸化可能在细胞骨架的形成中起关键作用。该分析确定了许多高度保守的蛋白质和磷酸化位点,它们主要参与葡萄糖代谢和蛋白质合成。S.eriocheiris。乙酰化和磷酸化的相对位置的比较表明,两个修饰通常位于非常接近同一蛋白质的位置。
结论:结果表明磷酸化在定义螺旋体功能状态中的作用是前所未有的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号