Current Proteomics ( IF 0.5 ) Pub Date : 2020-09-30 , DOI: 10.2174/1570164617666191206162237 Arundhati Banerjee 1 , Rakhi Dasgupta 1 , Sujay Ray 2
|
Background: Invasion of HIV in human occurs through DC-SIGN’s interaction via the mucosal lining during sexual transmission. Bovine Lactoferrin (bLF) has been known to hinder this invasion via its interaction with DC-SIGN. Hitherto, protein assays have taken place but molecular-level studies remain unexplored.
Methodology: The 3D structures of the three proteins were studied. After protein docking (bLF_DCSIGN and gp120_DC-SIGN), the complexes underwent simulation. Stability parameters and binding patterns with residues were explored.
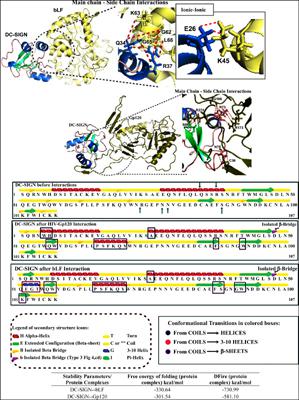
Results and Conclusion: ΔG values, net area for solvent accessibilities and conformational fluctuations in DC-SIGN affirm the binding of bLF with DC-SIGN to be more spontaneous and steadier contrary to that with gp120. Residue participation inferred more interactions to occur from bLF complex with a greater percentage of arginine (which strengthens the interaction) while electrostatic interaction between Lys45 (bLF) and Glu26 (DC-SIGN) strengthened the complex. Arg37 played an active role from DC-SIGN to form the stabilizing charged-neutral H-bond, while Lys63 from bLF formed two more such stabilizing charged-neutral H-bond with DC-SIGN. The prime binding sites in DC-SIGN; Arg37 and Gln34 occupy helices. The binding pockets in DC-SIGN may be blocked by bLF spontaneously, to hinder their interaction with gp120. No ionic-ionic interaction was observed from gp120_DCSIGN complex. 88th residue, which was a predominant residue in the binding pocket was found to experience a conformational shift from coils to sheets after interaction of DC-SIGN with bLF. This would instigate the pharmaceutical research as non-toxic LF would be economic as a remarkable peptide inhibitor opposing HIV.
中文翻译:

通过阻止DC-SIGN_GP120相互作用防止牛LF感染黏膜层的计算研究
背景:HIV感染是通过性传播过程中DC-SIGN通过粘膜内衬的相互作用而发生的。已知牛乳铁蛋白(bLF)通过与DC-SIGN相互作用来阻止这种侵袭。迄今为止,已经进行了蛋白质测定,但是尚未进行分子水平的研究。
方法:研究了三种蛋白质的3D结构。蛋白质对接后(bLF_DCSIGN和gp120_DC-SIGN),对复合物进行了模拟。探索了稳定性参数和与残基的结合模式。
结果与结论:ΔG值,溶剂可及性的净面积和DC-SIGN中的构象波动证实bLF与DC-SIGN的结合与gp120相比更加自发和稳定。残基的参与导致bLF配合物与精氨酸百分比更高(增强相互作用)的bLF配合物发生更多的相互作用,而Lys45(bLF)和Glu26(DC-SIGN)之间的静电相互作用增强了配合物。Arg37在DC-SIGN中起着积极的作用,形成稳定的带电中性H键,而bLF的Lys63在DC-SIGN的作用下又形成了两个这样的稳定的带电中性H键。DC-SIGN中的主要结合位点;Arg37和Gln34占据了螺旋。DC-SIGN中的结合口袋可能会被bLF自发阻断,以阻止它们与gp120的相互作用。从gp120_DCSIGN复合物中未观察到离子-离子相互作用。88在DC-SIGN与bLF相互作用后,发现第三残基(其为结合袋中的主要残基)经历了从卷曲到薄片的构象变化。这将刺激药物研究,因为无毒的LF作为对抗HIV的显着肽抑制剂非常经济。











































 京公网安备 11010802027423号
京公网安备 11010802027423号