当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible Protein Conjugation on Live Cell Surfaces by Specific Recognition between Coiled-Coil Motifs of Natural Amino Acid Sequences.
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-07-17 , DOI: 10.1021/acs.biomac.0c00569 Joon Hyung Ahn 1 , Sunah Kang 1 , Sohyun Park 1 , Hojoon Song 1 , Yaejin Yun 1 , Sejong Choi 1 , Seung-Eun Chong 1 , Dae Hee Cheon 1 , Dahyun Chun 1 , Jae Hoon Oh 1 , Sohee Nam 1 , Yan Lee 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-07-17 , DOI: 10.1021/acs.biomac.0c00569 Joon Hyung Ahn 1 , Sunah Kang 1 , Sohyun Park 1 , Hojoon Song 1 , Yaejin Yun 1 , Sejong Choi 1 , Seung-Eun Chong 1 , Dae Hee Cheon 1 , Dahyun Chun 1 , Jae Hoon Oh 1 , Sohee Nam 1 , Yan Lee 1
Affiliation
|
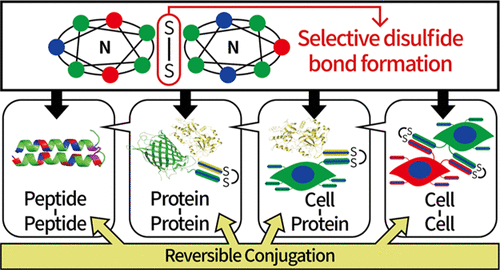
In this study, we propose a reversible covalent conjugation method for peptides, proteins, and even live cells based on specific recognition between natural amino acid sequences. Two heptad sequences can specifically recognize each other and induce the formation of a disulfide bond between cysteine residues. We show the covalent bond formation and dissociation between peptides and proteins in cell-free conditions and on the surface of live cells. Because heptad sequences consist of natural amino acids, they are expressed in cells without additional preparation and can be used to selectively conjugate peptides, proteins, and cells.
中文翻译:

通过天然氨基酸序列的螺旋线圈基序之间的特异性识别,在活细胞表面上发生可逆的蛋白质缀合。
在这项研究中,我们提出了一种基于天然氨基酸序列之间的特异性识别的肽,蛋白质甚至活细胞的可逆共价结合方法。两个七肽序列可以特异性识别彼此,并诱导半胱氨酸残基之间形成二硫键。我们展示了在无细胞条件下和活细胞表面上肽与蛋白质之间的共价键形成和解离。由于七肽序列由天然氨基酸组成,因此它们无需额外制备即可在细胞中表达,可用于选择性缀合肽,蛋白质和细胞。
更新日期:2020-09-14
中文翻译:

通过天然氨基酸序列的螺旋线圈基序之间的特异性识别,在活细胞表面上发生可逆的蛋白质缀合。
在这项研究中,我们提出了一种基于天然氨基酸序列之间的特异性识别的肽,蛋白质甚至活细胞的可逆共价结合方法。两个七肽序列可以特异性识别彼此,并诱导半胱氨酸残基之间形成二硫键。我们展示了在无细胞条件下和活细胞表面上肽与蛋白质之间的共价键形成和解离。由于七肽序列由天然氨基酸组成,因此它们无需额外制备即可在细胞中表达,可用于选择性缀合肽,蛋白质和细胞。











































 京公网安备 11010802027423号
京公网安备 11010802027423号