当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cognitive Improvement by Vorinostat through Modulation of Endoplasmic Reticulum Stress in a Corticosterone-Induced Chronic Stress Model in Mice.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-07-16 , DOI: 10.1021/acschemneuro.0c00315 Athira K V 1, 2 , Rajaram Mohanrao Madhana 1 , Akhilesh Kumar Bais 1 , Vijay Bahadur Singh 1 , Arpit Malik 1 , Swapnil Sinha 3 , Mangala Lahkar 4 , Pramod Kumar 5 , Pavan Kumar Samudrala 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-07-16 , DOI: 10.1021/acschemneuro.0c00315 Athira K V 1, 2 , Rajaram Mohanrao Madhana 1 , Akhilesh Kumar Bais 1 , Vijay Bahadur Singh 1 , Arpit Malik 1 , Swapnil Sinha 3 , Mangala Lahkar 4 , Pramod Kumar 5 , Pavan Kumar Samudrala 1
Affiliation
|
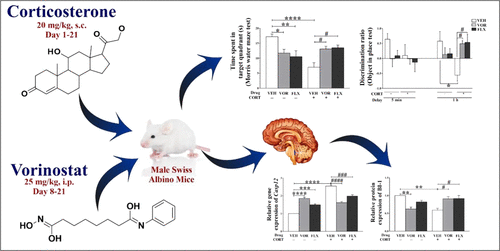
Chronic stress is the leading cause of memory impairment today. Various stress-based models are being developed for studying cognitive impairment. Repurposing of existing drugs in a new pharmacology class is the safest and cheapest option for treatment instead of new drug discovery. Vorinostat (VOR) is the first histone deacetylase (HDAC) inhibitor approved for the treatment of cutaneous T-cell lymphoma by the U.S. FDA. VOR follows the rule of five and is reported to cross the blood–brain barrier. Therefore, we aimed to evaluate the procognitive potential of VOR (25 mg/kg) administered by intraperitoneal (ip) route in a stress-based model of chronic corticosterone (CORT) injections (20 mg/kg, subcutaneously (sc)). The study comprised six groups. Normal mice were administered vehicle (VEH) (days 1–21, sc) in the first group, VOR (days 8–21, 25 mg/kg, ip) in the second group, and fluoxetine (FLX) (days 8–21, 15 mg/kg, oral) in the third group. Mice in the remaining three groups were given 20 mg/kg (sc) CORT for 21 days, and VOR (days 8–21, 25 mg/kg, ip) or FLX (days 8–21, 15 mg/kg, oral) was additionally administered to the treatment groups. Behavioral tests such as Morris water maze test, novel object recognition test, and object in place test were performed at the end of the dosing schedule to assess cognition. After behavior tests, mice were sacrificed, and hippocampus was separated from brain tissue for reverse transcriptase polymerase chain reaction (RT-PCR), Western blot, and immunohistochemistry studies. VOR treatment attenuated endoplasmic reticulum (ER) stress in CORT mice as evident from the reduction in DNA damage-inducible transcript 3 (Ddit3) (gene encoding CHOP), caspase 12 (Casp12), and calpain-2 (Capn2) mRNA levels, and cleaved caspase 3 (CASP3) protein expression. Bax inhibitor-1 (BI-1) was significantly increased in VOR-treated CORT mice. VOR also reversed CORT induced increase in HDAC2 level in the CA3 region. The protective effects of VOR were comparable to that of FLX in CORT mice. Thus, VOR has the potential to reverse cognitive dysfunction via modulation of ER stress markers and HDAC2.
中文翻译:

伏立诺他通过皮质类固醇诱导的小鼠慢性应激模型中内质网应激的调节改善认知能力。
慢性压力是当今记忆障碍的主要原因。正在开发各种基于压力的模型来研究认知障碍。在新药理学类别中重新利用现有药物是治疗新药而不是发现新药的最安全,最便宜的选择。伏立诺他(VOR)是第一种获美国FDA批准用于治疗皮肤T细胞淋巴瘤的组蛋白脱乙酰基酶(HDAC)抑制剂。VOR遵循5的规则,并据报道已穿越血脑屏障。因此,我们的目的是在基于压力的慢性皮质酮(CORT)注射模型(20 mg / kg,皮下注射(sc))中评估腹膜内(ip)途径给予VOR(25 mg / kg)的认知能力。该研究分为六组。在第一组中,正常小鼠接受了媒介物(VEH)(第1-21天,皮下注射),VOR(第8-21天,第二组为25 mg / kg,腹腔注射;第三组为氟西汀(FLX)(第8-21天,口服15 mg / kg)。其余三组的小鼠接受20 mg / kg(sc)CORT治疗21天,并给予VOR(8-21天,25 mg / kg,腹腔注射)或FLX(8-21天,15 mg / kg,口服)。另外向治疗组给药。在给药时间表结束时进行了行为测试,例如莫里斯水迷宫测试,新颖的物体识别测试和物体放置测试,以评估认知能力。行为测试后,处死小鼠,将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),Western印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(第三组为氟西汀(FLX)(第8-21天,口服15 mg / kg)。其余三组的小鼠接受20 mg / kg(sc)CORT治疗21天,并给予VOR(8-21天,25 mg / kg,腹腔注射)或FLX(8-21天,15 mg / kg,口服)。另外向治疗组给药。在给药时间表结束时进行了行为测试,例如莫里斯水迷宫测试,新颖的物体识别测试和物体放置测试,以评估认知能力。行为测试后,处死小鼠,将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),Western印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(第三组为氟西汀(FLX)(第8-21天,口服15 mg / kg)。其余三组的小鼠接受20 mg / kg(sc)CORT治疗21天,并给予VOR(8-21天,25 mg / kg,腹腔注射)或FLX(8-21天,15 mg / kg,口服)。另外向治疗组给药。在给药方案结束时进行了行为测试,例如莫里斯水迷宫测试,新颖的物体识别测试和就地物体测试,以评估认知度。行为测试后,处死小鼠,将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),Western印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(其余三组的小鼠接受20 mg / kg(sc)CORT治疗21天,并给予VOR(8-21天,25 mg / kg,腹腔注射)或FLX(8-21天,15 mg / kg,口服)。另外向治疗组给药。在给药时间表结束时进行了行为测试,例如莫里斯水迷宫测试,新颖的物体识别测试和物体放置测试,以评估认知能力。行为测试后,处死小鼠,将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),Western印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(其余三组的小鼠接受20 mg / kg(sc)CORT治疗21天,并给予VOR(8-21天,25 mg / kg,腹腔注射)或FLX(8-21天,15 mg / kg,口服)。另外向治疗组给药。在给药方案结束时进行了行为测试,例如莫里斯水迷宫测试,新颖的物体识别测试和就地物体测试,以评估认知度。行为测试后,处死小鼠,将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),Western印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(口服)另外给予治疗组。在给药方案结束时进行了行为测试,例如莫里斯水迷宫测试,新颖的物体识别测试和就地物体测试,以评估认知度。行为测试后,处死小鼠,将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),Western印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(口服)另外给予治疗组。在给药方案结束时进行了行为测试,例如莫里斯水迷宫测试,新颖的物体识别测试和物体放置测试,以评估认知能力。进行行为测试后,处死小鼠,将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),Western印迹和免疫组织化学研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),蛋白质印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),蛋白质印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(Ddit3)(编码CHOP的基因),caspase 12(Casp12)和calpain-2(Capn2)mRNA水平以及裂解的caspase 3(CASP3)蛋白表达。在VOR治疗的CORT小鼠中,Bax抑制剂1(BI-1)显着增加。VOR还逆转了CORT诱导的CA3区HDAC2水平升高。在CORT小鼠中,VOR的保护作用与FLX相当。因此,VOR具有通过调节ER应激标志物和HDAC2逆转认知功能障碍的潜力。
更新日期:2020-09-02
中文翻译:

伏立诺他通过皮质类固醇诱导的小鼠慢性应激模型中内质网应激的调节改善认知能力。
慢性压力是当今记忆障碍的主要原因。正在开发各种基于压力的模型来研究认知障碍。在新药理学类别中重新利用现有药物是治疗新药而不是发现新药的最安全,最便宜的选择。伏立诺他(VOR)是第一种获美国FDA批准用于治疗皮肤T细胞淋巴瘤的组蛋白脱乙酰基酶(HDAC)抑制剂。VOR遵循5的规则,并据报道已穿越血脑屏障。因此,我们的目的是在基于压力的慢性皮质酮(CORT)注射模型(20 mg / kg,皮下注射(sc))中评估腹膜内(ip)途径给予VOR(25 mg / kg)的认知能力。该研究分为六组。在第一组中,正常小鼠接受了媒介物(VEH)(第1-21天,皮下注射),VOR(第8-21天,第二组为25 mg / kg,腹腔注射;第三组为氟西汀(FLX)(第8-21天,口服15 mg / kg)。其余三组的小鼠接受20 mg / kg(sc)CORT治疗21天,并给予VOR(8-21天,25 mg / kg,腹腔注射)或FLX(8-21天,15 mg / kg,口服)。另外向治疗组给药。在给药时间表结束时进行了行为测试,例如莫里斯水迷宫测试,新颖的物体识别测试和物体放置测试,以评估认知能力。行为测试后,处死小鼠,将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),Western印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(第三组为氟西汀(FLX)(第8-21天,口服15 mg / kg)。其余三组的小鼠接受20 mg / kg(sc)CORT治疗21天,并给予VOR(8-21天,25 mg / kg,腹腔注射)或FLX(8-21天,15 mg / kg,口服)。另外向治疗组给药。在给药时间表结束时进行了行为测试,例如莫里斯水迷宫测试,新颖的物体识别测试和物体放置测试,以评估认知能力。行为测试后,处死小鼠,将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),Western印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(第三组为氟西汀(FLX)(第8-21天,口服15 mg / kg)。其余三组的小鼠接受20 mg / kg(sc)CORT治疗21天,并给予VOR(8-21天,25 mg / kg,腹腔注射)或FLX(8-21天,15 mg / kg,口服)。另外向治疗组给药。在给药方案结束时进行了行为测试,例如莫里斯水迷宫测试,新颖的物体识别测试和就地物体测试,以评估认知度。行为测试后,处死小鼠,将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),Western印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(其余三组的小鼠接受20 mg / kg(sc)CORT治疗21天,并给予VOR(8-21天,25 mg / kg,腹腔注射)或FLX(8-21天,15 mg / kg,口服)。另外向治疗组给药。在给药时间表结束时进行了行为测试,例如莫里斯水迷宫测试,新颖的物体识别测试和物体放置测试,以评估认知能力。行为测试后,处死小鼠,将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),Western印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(其余三组的小鼠接受20 mg / kg(sc)CORT治疗21天,并给予VOR(8-21天,25 mg / kg,腹腔注射)或FLX(8-21天,15 mg / kg,口服)。另外向治疗组给药。在给药方案结束时进行了行为测试,例如莫里斯水迷宫测试,新颖的物体识别测试和就地物体测试,以评估认知度。行为测试后,处死小鼠,将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),Western印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(口服)另外给予治疗组。在给药方案结束时进行了行为测试,例如莫里斯水迷宫测试,新颖的物体识别测试和就地物体测试,以评估认知度。行为测试后,处死小鼠,将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),Western印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(口服)另外给予治疗组。在给药方案结束时进行了行为测试,例如莫里斯水迷宫测试,新颖的物体识别测试和物体放置测试,以评估认知能力。进行行为测试后,处死小鼠,将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),Western印迹和免疫组织化学研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),蛋白质印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(将海马从脑组织中分离出来,用于逆转录聚合酶链反应(RT-PCR),蛋白质印迹和免疫组化研究。VOR处理可减轻CORT小鼠的内质网(ER)应激,这可从DNA损伤诱导性转录本3(Ddit3)(编码CHOP的基因),caspase 12(Casp12)和calpain-2(Capn2)mRNA水平以及裂解的caspase 3(CASP3)蛋白表达。在VOR治疗的CORT小鼠中,Bax抑制剂1(BI-1)显着增加。VOR还逆转了CORT诱导的CA3区HDAC2水平升高。在CORT小鼠中,VOR的保护作用与FLX相当。因此,VOR具有通过调节ER应激标志物和HDAC2逆转认知功能障碍的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号