当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Decarboxylative C(sp3)−N Bond Forming Reaction to Construct 4‐Imidazolidinones via Post‐Ugi Cascade Sequence in One Pot
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-07-17 , DOI: 10.1002/adsc.202000736 Gui‐Ting Song 1 , Chuan‐Hua Qu 1 , Jie Lei 1, 2 , Wei Yan 2 , Dian‐Yong Tang 1 , Hong‐yu Li 2 , Zhong‐Zhu Chen 1 , Zhi‐Gang Xu 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-07-17 , DOI: 10.1002/adsc.202000736 Gui‐Ting Song 1 , Chuan‐Hua Qu 1 , Jie Lei 1, 2 , Wei Yan 2 , Dian‐Yong Tang 1 , Hong‐yu Li 2 , Zhong‐Zhu Chen 1 , Zhi‐Gang Xu 1
Affiliation
|
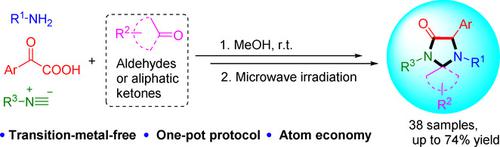
A post‐Ugi one‐pot cascade was developed to access 4‐imidazolidinones through an intramolecular decarboxylative C(sp3)−N bond forming reaction. The reaction has a broad tolerance for a variety of substituted aldehydes, anilines, isocyanides and glyoxylic acids. The cascade reaction scope was expanded to synthesize spiroimidazolidinone by the replacement of aldehyde with aliphatic ketone in the Ugi reaction. Subsequently, the methodology was applied to synthesize the core structures of pharmaceuticals GSK2137305 and SCH 900822 under the mild and facile conditions with one purification. This cascade reaction generates opportunities for the tailored synthesis of a range of biologically active 4‐imidazolidinones through tuneable Ugi inputs.
中文翻译:

通过一锅中的Ugi后级联序列对构建4-咪唑啉酮的脱羧化C(sp3)-N键形成反应
Ugi后的一锅级联反应是通过分子内脱羧化C(sp 3)-N键形成反应来获得4-咪唑啉酮的。该反应对各种取代的醛,苯胺,异氰酸酯和乙醛酸具有宽泛的耐受性。通过在Ugi反应中用脂肪族酮取代醛,扩大了级联反应的范围,以合成螺氨基咪唑啉酮。随后,将该方法应用于在温和和简便条件下一次纯化即可合成药物GSK2137305和SCH 900822的核心结构。这种级联反应为通过可调节的Ugi输入量身定制一系列具有生物活性的4-咪唑啉酮提供了机会。
更新日期:2020-07-17
中文翻译:

通过一锅中的Ugi后级联序列对构建4-咪唑啉酮的脱羧化C(sp3)-N键形成反应
Ugi后的一锅级联反应是通过分子内脱羧化C(sp 3)-N键形成反应来获得4-咪唑啉酮的。该反应对各种取代的醛,苯胺,异氰酸酯和乙醛酸具有宽泛的耐受性。通过在Ugi反应中用脂肪族酮取代醛,扩大了级联反应的范围,以合成螺氨基咪唑啉酮。随后,将该方法应用于在温和和简便条件下一次纯化即可合成药物GSK2137305和SCH 900822的核心结构。这种级联反应为通过可调节的Ugi输入量身定制一系列具有生物活性的4-咪唑啉酮提供了机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号