当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and synthesis of new series of 3‐cyanopyridine and pyrazolopyridine derivatives
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-07-15 , DOI: 10.1002/jhet.4058 Reda Mohammed Keshk 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-07-15 , DOI: 10.1002/jhet.4058 Reda Mohammed Keshk 1
Affiliation
|
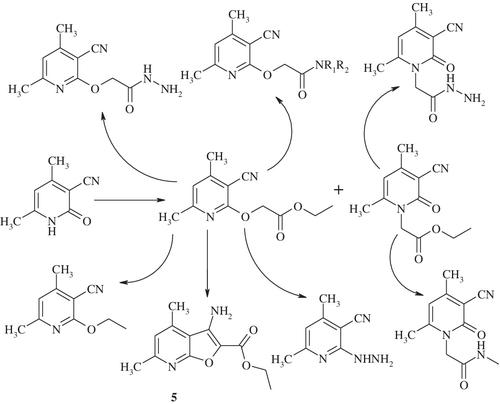
Reaction of 3‐cyano‐4,6‐dimethyl‐2‐pyridone with ethyl chloroacetate afforded ethyl 2‐([3‐cyano‐4,6‐dimethylpyridin‐2‐yl]oxy)acetate 2 and ethyl 2‐(3‐cyano‐4,6‐dimethyl‐2‐oxopyridin‐1[2H]‐yl)acetate 3, the reaction product yield depend on the reaction condition (potassium carbonate concentration and reaction time). These compounds used as precursors to synthesize pyridine derivatives 4, 6‐10, 15, 17‐20, furopyridines 5, 16, pyrazolopyridine 12, pyridopyrazolopyrimidines 14a,b. The structure of the newly synthesized compounds was confirmed by spectral data (IR, NMR, and mass spectra) and elemental analysis.
中文翻译:

新系列3-氰基吡啶和吡唑并吡啶衍生物的设计与合成
3-氰基-4-6,6-二甲基-2-吡啶酮与氯乙酸乙酯的反应生成2-([3-氰基-4,6-二甲基吡啶-2-基]氧基)乙酸乙酯2和2-(3-氰基乙基)乙酸乙酯-4,6-二甲基-2-氧杂吡啶-1 [2 H ]-基)乙酸酯3,反应产物的产率取决于反应条件(碳酸钾浓度和反应时间)。用作前体以合成吡啶衍生物。这些化合物4,6-10,15,17-20,呋喃并吡啶5,16,吡唑并吡啶12,pyridopyrazolopyrimidines 14A,B。通过光谱数据(IR,NMR和质谱)和元素分析确认了新合成化合物的结构。
更新日期:2020-09-08
中文翻译:

新系列3-氰基吡啶和吡唑并吡啶衍生物的设计与合成
3-氰基-4-6,6-二甲基-2-吡啶酮与氯乙酸乙酯的反应生成2-([3-氰基-4,6-二甲基吡啶-2-基]氧基)乙酸乙酯2和2-(3-氰基乙基)乙酸乙酯-4,6-二甲基-2-氧杂吡啶-1 [2 H ]-基)乙酸酯3,反应产物的产率取决于反应条件(碳酸钾浓度和反应时间)。用作前体以合成吡啶衍生物。这些化合物4,6-10,15,17-20,呋喃并吡啶5,16,吡唑并吡啶12,pyridopyrazolopyrimidines 14A,B。通过光谱数据(IR,NMR和质谱)和元素分析确认了新合成化合物的结构。









































 京公网安备 11010802027423号
京公网安备 11010802027423号