当前位置:
X-MOL 学术
›
ChemNanoMat
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Anticancer Therapeutic Delivery to Tumor Microenvironment via Simultaneous Angiogenesis and Tumor Targeting
ChemNanoMat ( IF 2.6 ) Pub Date : 2020-07-17 , DOI: 10.1002/cnma.202000309 Yongli Zhang 1 , Sihan Wang 2, 3 , Haixia Wang 3 , Yingjie Yang 4 , Jin Gao 3
ChemNanoMat ( IF 2.6 ) Pub Date : 2020-07-17 , DOI: 10.1002/cnma.202000309 Yongli Zhang 1 , Sihan Wang 2, 3 , Haixia Wang 3 , Yingjie Yang 4 , Jin Gao 3
Affiliation
|
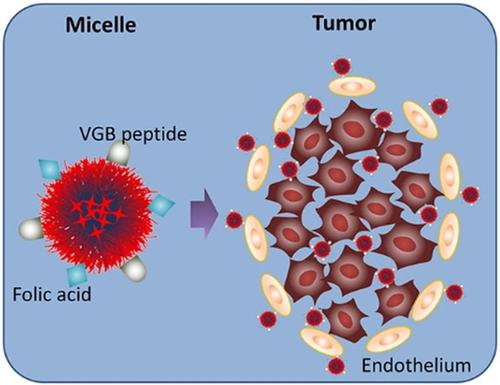
Angiogenesis is an event commonly occurring during tumorigenesis and the following development and progression provide an ideal target for anticancer drug‐targeted delivery. Nanoparticles with tumor‐cell binding ability are usually used for tumor therapy and proved to be able to accumulate in tumor tissues. The nanoparticles with dual angiogenesis and tumor‐cell targeting capacity are expected to deliver therapeutics to the lesions of tumors more efficiently and should achieve a better tumor therapeutical effect. In the present study, we employed melanoma xenograft as a tumor model, with VGB peptide and FA as angiogenesis and tumor cell guiding moieties, and with micelles as a model nanoparticle to examine our hypothesis. As a result, we proved that the dual strategy enhanced the delivery of bufalin, an anticancer agent, and achieved a better therapeutical outcome for melanoma, compared to the conventional single‐targeting methods. Since angiogenesis is common in tumor development, the simultaneous targeting strategy can be flexibly adapted to a wide range of tumors.
中文翻译:

通过同时血管生成和肿瘤靶向增强的抗癌治疗药物向肿瘤微环境的传递。
血管生成是在肿瘤发生期间通常发生的事件,随后的发展和进程为抗癌药物靶向递送提供了理想的靶标。具有肿瘤细胞结合能力的纳米粒子通常用于肿瘤治疗,并被证明能够在肿瘤组织中积累。具有双重血管生成和肿瘤细胞靶向能力的纳米粒子有望更有效地将治疗剂提供给肿瘤病变,并应达到更好的肿瘤治疗效果。在本研究中,我们采用黑色素瘤异种移植作为肿瘤模型,以VGB肽和FA为血管生成和肿瘤细胞指导部分,并以胶束作为模型纳米颗粒来检验我们的假设。结果,我们证明了双重策略可增强抗癌药bufalin的递送,与传统的单一靶向方法相比,黑色素瘤具有更好的治疗效果。由于血管生成在肿瘤发展中很常见,因此同时靶向策略可以灵活地适应多种肿瘤。
更新日期:2020-07-17
中文翻译:

通过同时血管生成和肿瘤靶向增强的抗癌治疗药物向肿瘤微环境的传递。
血管生成是在肿瘤发生期间通常发生的事件,随后的发展和进程为抗癌药物靶向递送提供了理想的靶标。具有肿瘤细胞结合能力的纳米粒子通常用于肿瘤治疗,并被证明能够在肿瘤组织中积累。具有双重血管生成和肿瘤细胞靶向能力的纳米粒子有望更有效地将治疗剂提供给肿瘤病变,并应达到更好的肿瘤治疗效果。在本研究中,我们采用黑色素瘤异种移植作为肿瘤模型,以VGB肽和FA为血管生成和肿瘤细胞指导部分,并以胶束作为模型纳米颗粒来检验我们的假设。结果,我们证明了双重策略可增强抗癌药bufalin的递送,与传统的单一靶向方法相比,黑色素瘤具有更好的治疗效果。由于血管生成在肿瘤发展中很常见,因此同时靶向策略可以灵活地适应多种肿瘤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号