Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Accelerated Amyloid Beta Pathogenesis by Bacterial Amyloid FapC
Advanced Science ( IF 14.3 ) Pub Date : 2020-07-16 , DOI: 10.1002/advs.202001299 Ibrahim Javed 1, 2 , Zhenzhen Zhang 3 , Jozef Adamcik 4 , Nicholas Andrikopoulos 2 , Yuhuan Li 2 , Daniel E Otzen 5 , Sijie Lin 6 , Raffaele Mezzenga 4 , Thomas P Davis 1, 2 , Feng Ding 3 , Pu Chun Ke 2, 7
Advanced Science ( IF 14.3 ) Pub Date : 2020-07-16 , DOI: 10.1002/advs.202001299 Ibrahim Javed 1, 2 , Zhenzhen Zhang 3 , Jozef Adamcik 4 , Nicholas Andrikopoulos 2 , Yuhuan Li 2 , Daniel E Otzen 5 , Sijie Lin 6 , Raffaele Mezzenga 4 , Thomas P Davis 1, 2 , Feng Ding 3 , Pu Chun Ke 2, 7
Affiliation
|
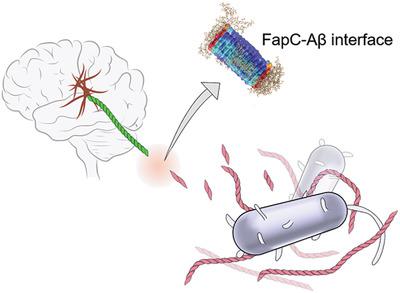
The gut–brain axis has attracted increasing attention in recent years, fueled by accumulating symptomatic, physiological, and pathological findings. In this study, the aggregation and toxicity of amyloid beta (Aβ), the pathogenic peptide associated with Alzheimer's disease (AD), seeded by FapC amyloid fragments (FapCS) of Pseudomonas aeruginosa that colonizes the gut microbiome through infections are examined. FapCS display favorable binding with Aβ and a catalytic capacity in seeding the peptide amyloidosis. Upon seeding, twisted Aβ fibrils assume a much‐shortened periodicity approximating that of FapC fibrils, accompanied by a 37% sharp rise in the fibrillar diameter, compared with the control. The robust seeding capacity for Aβ by FapCS and the biofilm fragments derived from P. aeruginosa entail abnormal behavior pathology and immunohistology, as well as impaired cognitive function of zebrafish. Together, the data offer the first concrete evidence of structural integration and inheritance in peptide cross‐seeding, a crucial knowledge gap in understanding the pathological correlations between different amyloid diseases. The catalytic role of infectious bacteria in promoting Aβ amyloidosis may be exploited as a potential therapeutic target, while the altered mesoscopic signatures of Aβ fibrils may serve as a prototype for molecular assembly and a biomarker for screening bacterial infections in AD.
中文翻译:

细菌淀粉样蛋白 FapC 加速β淀粉样蛋白发病机制
近年来,由于症状、生理和病理学发现的积累,肠-脑轴引起了越来越多的关注。在这项研究中,研究了β淀粉样蛋白( Aβ )的聚集和毒性,β淀粉样蛋白是与阿尔茨海默病(AD)相关的致病肽,由铜绿假单胞菌的FapC淀粉样蛋白片段(FapCS)播种,通过感染在肠道微生物组中定殖。 FapCS 表现出与 A β 的良好结合以及促进肽淀粉样变性的催化能力。接种后,与对照相比,扭曲的 A β原纤维的周期性大大缩短,接近 FapC 原纤维的周期,同时原纤维直径急剧增加 37%。 FapCS 和铜绿假单胞菌衍生的生物膜片段对 A β 的强大播种能力导致斑马鱼行为病理学和免疫组织学异常,以及认知功能受损。总之,这些数据提供了肽交叉播种中结构整合和遗传的第一个具体证据,这是理解不同淀粉样蛋白疾病之间病理相关性的关键知识差距。感染性细菌在促进 A β淀粉样变性中的催化作用可作为潜在的治疗靶点,而 A β原纤维改变的介观特征可作为分子组装的原型和筛选 AD 细菌感染的生物标志物。
更新日期:2020-09-23
中文翻译:

细菌淀粉样蛋白 FapC 加速β淀粉样蛋白发病机制
近年来,由于症状、生理和病理学发现的积累,肠-脑轴引起了越来越多的关注。在这项研究中,研究了β淀粉样蛋白( Aβ )的聚集和毒性,β淀粉样蛋白是与阿尔茨海默病(AD)相关的致病肽,由铜绿假单胞菌的FapC淀粉样蛋白片段(FapCS)播种,通过感染在肠道微生物组中定殖。 FapCS 表现出与 A β 的良好结合以及促进肽淀粉样变性的催化能力。接种后,与对照相比,扭曲的 A β原纤维的周期性大大缩短,接近 FapC 原纤维的周期,同时原纤维直径急剧增加 37%。 FapCS 和铜绿假单胞菌衍生的生物膜片段对 A β 的强大播种能力导致斑马鱼行为病理学和免疫组织学异常,以及认知功能受损。总之,这些数据提供了肽交叉播种中结构整合和遗传的第一个具体证据,这是理解不同淀粉样蛋白疾病之间病理相关性的关键知识差距。感染性细菌在促进 A β淀粉样变性中的催化作用可作为潜在的治疗靶点,而 A β原纤维改变的介观特征可作为分子组装的原型和筛选 AD 细菌感染的生物标志物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号