当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Recovery and usage of CO2 produced in the Fischer-Tropsch synthesis employing Gibbs energy minimization
Fluid Phase Equilibria ( IF 2.6 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.fluid.2020.112678 Fernando Henrique Marques , Reginaldo Guirardello
Fluid Phase Equilibria ( IF 2.6 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.fluid.2020.112678 Fernando Henrique Marques , Reginaldo Guirardello
|
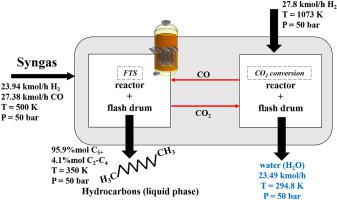
Abstract This paper proposes a process to recover and recycle the carbon dioxide produced during Fischer-Tropsch synthesis (FTS) through thermodynamic analysis. Thus, it may avoid the release of large amounts of CO2 to the atmosphere. We investigated the chemical and phase equilibrium of Fischer-Tropsch reaction using C O 2 as a reactant. Also, the chemical equilibrium of reverse water-gas shift (RWGS) reaction was considered as an alternative to obtaining carbon monoxide overall process. The effects of temperature, pressure, and the molar ratio of reactants were evaluated at thermodynamic equilibrium using Gibbs energy minimization. The Gibbs energy minimization problem is formulated as mathematical optimization (objective function), and to represent the non-ideality of the system, we used the cubic equation of state (Soave-Redlich-Kwong) as constraints. Non-negativity of the number of moles and stoichiometric balances were considered as constraints, using the reactions that take into account the effect of the catalyst. The software GAMS with solver CONOPT is employed to solve this nonlinear programming problem. In FTS, adding C O 2 as co-reactant decreased the hydrocarbons production and has not presented an effective C O 2 conversion. Alternatively, RWGS showed a higher C O 2 conversion. Hence, it was possible to achieve a process in which C O 2 generated in FTS could be recovered from liquid hydrocarbons using a flash drum, and then converting it in carbon monoxide by RWGS reaction at high temperatures. The proposed process operate at the same pressure in all vessels, and only heat exchangers and flash drums are used to separate and recover C O 2 from the other compounds.
中文翻译:

采用吉布斯能量最小化的费托合成中产生的 CO2 的回收和使用
摘要 本文提出了一种通过热力学分析回收和回收费托合成(FTS)过程中产生的二氧化碳的方法。因此,它可以避免向大气中释放大量二氧化碳。我们研究了使用 CO 2 作为反应物的费托反应的化学和相平衡。此外,反向水煤气变换 (RWGS) 反应的化学平衡被认为是获得一氧化碳整个过程的替代方法。温度、压力和反应物摩尔比的影响在热力学平衡下使用吉布斯能量最小化进行评估。Gibbs 能量最小化问题被公式化为数学优化(目标函数),并表示系统的非理想性,我们使用三次状态方程 (Soave-Redlich-Kwong) 作为约束。使用考虑催化剂效应的反应,摩尔数和化学计量平衡的非负性被认为是约束条件。带有求解器 CONOPT 的软件 GAMS 被用来解决这个非线性规划问题。在 FTS 中,添加 CO 2 作为共反应物会降低碳氢化合物的产量,并且没有表现出有效的 CO 2 转化率。或者,RWGS 显示出更高的 CO 2 转化率。因此,有可能实现这样一种工艺,其中可以使用闪蒸罐从液态烃中回收 FTS 中产生的 CO 2,然后在高温下通过 RWGS 反应将其转化为一氧化碳。提议的过程在所有容器中以相同的压力运行,
更新日期:2020-11-01
中文翻译:

采用吉布斯能量最小化的费托合成中产生的 CO2 的回收和使用
摘要 本文提出了一种通过热力学分析回收和回收费托合成(FTS)过程中产生的二氧化碳的方法。因此,它可以避免向大气中释放大量二氧化碳。我们研究了使用 CO 2 作为反应物的费托反应的化学和相平衡。此外,反向水煤气变换 (RWGS) 反应的化学平衡被认为是获得一氧化碳整个过程的替代方法。温度、压力和反应物摩尔比的影响在热力学平衡下使用吉布斯能量最小化进行评估。Gibbs 能量最小化问题被公式化为数学优化(目标函数),并表示系统的非理想性,我们使用三次状态方程 (Soave-Redlich-Kwong) 作为约束。使用考虑催化剂效应的反应,摩尔数和化学计量平衡的非负性被认为是约束条件。带有求解器 CONOPT 的软件 GAMS 被用来解决这个非线性规划问题。在 FTS 中,添加 CO 2 作为共反应物会降低碳氢化合物的产量,并且没有表现出有效的 CO 2 转化率。或者,RWGS 显示出更高的 CO 2 转化率。因此,有可能实现这样一种工艺,其中可以使用闪蒸罐从液态烃中回收 FTS 中产生的 CO 2,然后在高温下通过 RWGS 反应将其转化为一氧化碳。提议的过程在所有容器中以相同的压力运行,



























 京公网安备 11010802027423号
京公网安备 11010802027423号