当前位置:
X-MOL 学术
›
Chem. Pap.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
(E)-N-(pyren-1-ylmethylene)benzenamine: efficient promoter for additive-free palladium catalyzed aerobic oxidative coupling of arylboronic acids and terminal alkynes
Chemical Papers ( IF 2.1 ) Pub Date : 2020-04-13 , DOI: 10.1007/s11696-020-01156-8 Jagadeesan Lakshmipraba , Rupesh Narayana Prabhu , Victor Violet Dhayabaran
中文翻译:

(E)-N-(吡喃-1-基亚甲基)苯甲胺:芳基硼酸和末端炔烃的无添加剂钯催化的需氧氧化偶联的有效促进剂

Chemical Papers ( IF 2.1 ) Pub Date : 2020-04-13 , DOI: 10.1007/s11696-020-01156-8 Jagadeesan Lakshmipraba , Rupesh Narayana Prabhu , Victor Violet Dhayabaran
Abstract
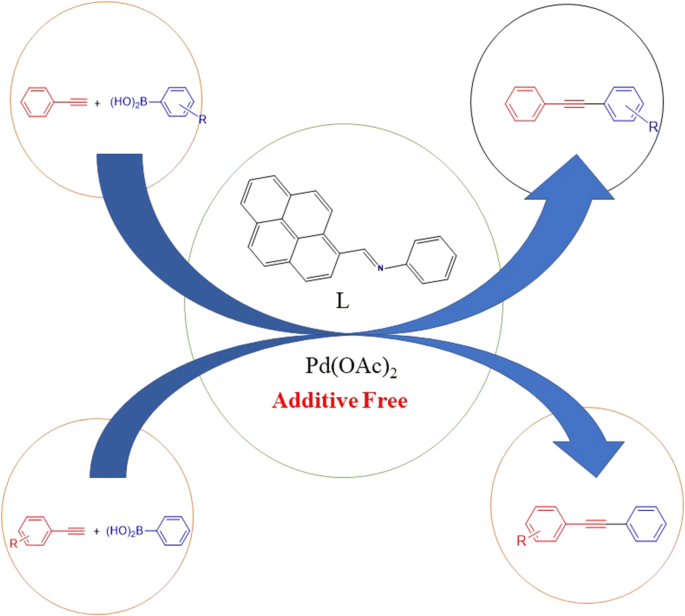
A highly productive protocol for the synthesis of internal alkynes by the carbon–carbon cross-coupling reactions of electronically different arylboronic acids with substituted phenylacetylenes was described by employing (E)-N-(pyren-1-ylmethylene)benzenamine with Pd(OAc)2. The influence of reaction parameters such as solvent, base and reaction temperature in this carbon–carbon cross-coupling reaction was also investigated. The substrate scope could be expanded to electron-poor alkynes, for which the conventional Sonogashira reaction gives poor yields. Moderate to excellent yield was obtained in the oxidative Sonogashira-type coupling reaction.Graphic abstract

中文翻译:

(E)-N-(吡喃-1-基亚甲基)苯甲胺:芳基硼酸和末端炔烃的无添加剂钯催化的需氧氧化偶联的有效促进剂
摘要
通过使用(E)-N-(吡喃-1-基亚甲基)苯胺与Pd(OAc)来描述通过电子不同的芳基硼酸与取代的苯基乙炔的碳-碳交叉偶联反应合成内部炔的高效方法2。还研究了反应参数(如溶剂,碱和反应温度)在该碳-碳交叉偶联反应中的影响。底物的范围可以扩大到电子贫乏的炔烃,常规的Sonogashira反应收率较差。在氧化Sonogashira型偶联反应中获得了中等至优异的产率。图形摘要












































 京公网安备 11010802027423号
京公网安备 11010802027423号