当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Formation of Deep Eutectic Solvents: Betaine as a Universal Hydrogen Bond Acceptor.
ChemSusChem ( IF 7.5 ) Pub Date : 2020-07-16 , DOI: 10.1002/cssc.202001331 Dinis O Abranches 1 , Liliana P Silva 1 , Mónia A R Martins 1 , Simão P Pinho 2 , João A P Coutinho 1
ChemSusChem ( IF 7.5 ) Pub Date : 2020-07-16 , DOI: 10.1002/cssc.202001331 Dinis O Abranches 1 , Liliana P Silva 1 , Mónia A R Martins 1 , Simão P Pinho 2 , João A P Coutinho 1
Affiliation
|
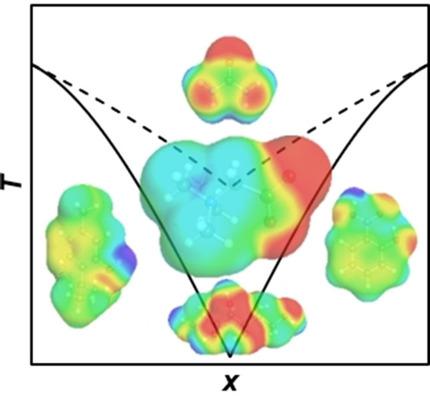
The mechanism of formation of betaine‐based deep eutectic solvents (DES) is presented for the first time. Due to its polarity unbalance, it was found that betaine displays strong negative deviations from ideality when mixed with a variety of different organic substances. These results pave the way for a comprehensive design of novel deep eutectic solvents. A connection to biologically relevant systems was made using betaine (osmolyte) and urea (protein denaturant), showing that these two compounds formed a DES, the molecular interactions of which were greatly enhanced in the presence of water.
中文翻译:

了解深共晶溶剂的形成:甜菜碱作为通用氢键受体。
首次介绍了基于甜菜碱的深共熔溶剂(DES)的形成机理。由于其极性不平衡,发现甜菜碱在与多种不同的有机物质混合时会表现出与理想情况强烈的负偏差。这些结果为新型深共晶溶剂的全面设计铺平了道路。使用甜菜碱(渗透液)和尿素(蛋白质变性剂)建立了与生物学相关系统的连接,表明这两种化合物形成了DES,在水的存在下其分子相互作用大大增强。
更新日期:2020-09-24
中文翻译:

了解深共晶溶剂的形成:甜菜碱作为通用氢键受体。
首次介绍了基于甜菜碱的深共熔溶剂(DES)的形成机理。由于其极性不平衡,发现甜菜碱在与多种不同的有机物质混合时会表现出与理想情况强烈的负偏差。这些结果为新型深共晶溶剂的全面设计铺平了道路。使用甜菜碱(渗透液)和尿素(蛋白质变性剂)建立了与生物学相关系统的连接,表明这两种化合物形成了DES,在水的存在下其分子相互作用大大增强。










































 京公网安备 11010802027423号
京公网安备 11010802027423号