当前位置:
X-MOL 学术
›
Vib. Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chitosan stabilization and control over hot spot formation of gold nanospheres and SERS performance evaluation
Vibrational Spectroscopy ( IF 2.7 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.vibspec.2020.103119 Débora G. de Oliveira , Giovana A. Pimentel , Gustavo F.S. Andrade
Vibrational Spectroscopy ( IF 2.7 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.vibspec.2020.103119 Débora G. de Oliveira , Giovana A. Pimentel , Gustavo F.S. Andrade
|
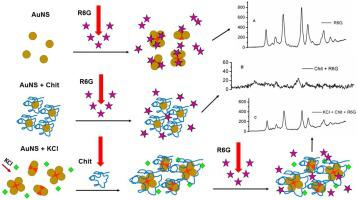
Abstract Au nanoparticles tend to aggregate in solution in the presence of several agents, specifically most adsorbates used in SERS experiments, such as rhodamine-6 G (R6 G). That tendency may be circumvented by stabilizing agents, usually based on modulating electrostatic interactions. The control over stability and formation of SERS hot spots are important factors for application in SERS-based biosensors. Among the stabilizing agents, chitosan (Chit), which is positively charged biopolymer, can provide stability to the AuNS system and control the formation of hot spots. In the present study, Au nanospheres (AuNS) were coated by Chit, which improved stability against KCl and ethanol. The decrease in R6 G SERS intensity relative to the unprotected AuNS was attributed to prevention of hot spots formation by the biopolymer coating; this last characteristic was partially reverted by aggregating with chloride before adding Chit. The AuNS + KCl + Chit system resulted in half R6 G SERS intensity compared to uncoated AuNS, but in an increase in 5 times compared to coating with Chit without the chloride-generated hot spots. Adding Chit improved the temporal stability of AuNS suspension for several weeks. The present study shows that Chit may both stabilize existing hot spots and stop the increase of aggregates, avoiding additional aggregation and agglomeration of Au nanoparticles even in presence of aggregating agents and still resulting in high SERS performance.
中文翻译:

壳聚糖稳定和控制金纳米球热点形成和 SERS 性能评估
摘要 Au 纳米粒子在存在多种试剂的情况下倾向于在溶液中聚集,特别是 SERS 实验中使用的大多数吸附物,如罗丹明 6 G (R6 G)。这种趋势可以通过稳定剂来规避,通常基于调节静电相互作用。SERS 热点的稳定性和形成的控制是在基于 SERS 的生物传感器中应用的重要因素。在稳定剂中,壳聚糖 (Chit) 是带正电荷的生物聚合物,可以为 AuNS 系统提供稳定性并控制热点的形成。在本研究中,金纳米球 (AuNS) 被 Chit 包覆,这提高了对 KCl 和乙醇的稳定性。相对于未受保护的 AuNS,R6 G SERS 强度的降低归因于生物聚合物涂层防止了热点的形成;通过在添加 Chit 之前与氯化物聚集,最后一个特性被部分恢复。与未涂覆的 AuNS 相比,AuNS + KCl + Chit 系统导致 R6 G SERS 强度的一半,但与没有氯化物产生的热点的 Chit 涂层相比增加了 5 倍。添加 Chit 提高了 AuNS 悬浮液的时间稳定性数周。目前的研究表明,Chit 既可以稳定现有的热点,又可以阻止聚集体的增加,即使在存在聚集剂的情况下也能避免 Au 纳米粒子的额外聚集和团聚,并且仍然具有较高的 SERS 性能。但与没有氯化物产生的热点的 Chit 涂层相比,增加了 5 倍。添加 Chit 提高了 AuNS 悬浮液的时间稳定性数周。目前的研究表明,Chit 既可以稳定现有的热点,又可以阻止聚集体的增加,即使在存在聚集剂的情况下也能避免 Au 纳米颗粒的额外聚集和团聚,并且仍能产生高 SERS 性能。但与没有氯化物产生的热点的 Chit 涂层相比,增加了 5 倍。添加 Chit 提高了 AuNS 悬浮液的时间稳定性数周。目前的研究表明,Chit 既可以稳定现有的热点,又可以阻止聚集体的增加,即使在存在聚集剂的情况下也能避免 Au 纳米颗粒的额外聚集和团聚,并且仍能产生高 SERS 性能。
更新日期:2020-09-01
中文翻译:

壳聚糖稳定和控制金纳米球热点形成和 SERS 性能评估
摘要 Au 纳米粒子在存在多种试剂的情况下倾向于在溶液中聚集,特别是 SERS 实验中使用的大多数吸附物,如罗丹明 6 G (R6 G)。这种趋势可以通过稳定剂来规避,通常基于调节静电相互作用。SERS 热点的稳定性和形成的控制是在基于 SERS 的生物传感器中应用的重要因素。在稳定剂中,壳聚糖 (Chit) 是带正电荷的生物聚合物,可以为 AuNS 系统提供稳定性并控制热点的形成。在本研究中,金纳米球 (AuNS) 被 Chit 包覆,这提高了对 KCl 和乙醇的稳定性。相对于未受保护的 AuNS,R6 G SERS 强度的降低归因于生物聚合物涂层防止了热点的形成;通过在添加 Chit 之前与氯化物聚集,最后一个特性被部分恢复。与未涂覆的 AuNS 相比,AuNS + KCl + Chit 系统导致 R6 G SERS 强度的一半,但与没有氯化物产生的热点的 Chit 涂层相比增加了 5 倍。添加 Chit 提高了 AuNS 悬浮液的时间稳定性数周。目前的研究表明,Chit 既可以稳定现有的热点,又可以阻止聚集体的增加,即使在存在聚集剂的情况下也能避免 Au 纳米粒子的额外聚集和团聚,并且仍然具有较高的 SERS 性能。但与没有氯化物产生的热点的 Chit 涂层相比,增加了 5 倍。添加 Chit 提高了 AuNS 悬浮液的时间稳定性数周。目前的研究表明,Chit 既可以稳定现有的热点,又可以阻止聚集体的增加,即使在存在聚集剂的情况下也能避免 Au 纳米颗粒的额外聚集和团聚,并且仍能产生高 SERS 性能。但与没有氯化物产生的热点的 Chit 涂层相比,增加了 5 倍。添加 Chit 提高了 AuNS 悬浮液的时间稳定性数周。目前的研究表明,Chit 既可以稳定现有的热点,又可以阻止聚集体的增加,即使在存在聚集剂的情况下也能避免 Au 纳米颗粒的额外聚集和团聚,并且仍能产生高 SERS 性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号