Structure ( IF 5.7 ) Pub Date : 2020-07-16 , DOI: 10.1016/j.str.2020.06.009 Christina M Zimanyi 1 , Mo Guo 1 , Arshad Mahmood 1 , Wayne A Hendrickson 2 , David Hirsh 2 , Jonah Cheung 1
|
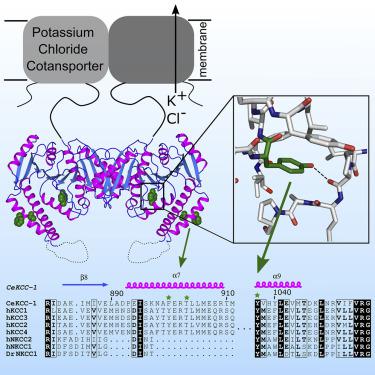
Cation-chloride cotransporters (CCCs) regulate the movement of chloride across membranes, controlling physiological processes from cell volume maintenance to neuronal signaling. Human CCCs are clinical targets for existing diuretics and potentially additional indications. Here, we report the X-ray crystal structure of the soluble C-terminal regulatory domain of a eukaryotic potassium-chloride cotransporter, Caenorhabditis elegans KCC-1. We observe a core α/β fold conserved among CCCs. Using structure-based sequence alignment, we analyze similarities and differences to the C-terminal domains of other CCC family members. We find that important regulatory motifs are in less-structured regions and residues important for dimerization are not widely conserved, suggesting that oligomerization and its effects may vary within the larger family. This snapshot of a eukaryotic KCC is a valuable starting point for the rational design of studies of cellular chloride regulation.
中文翻译:

真核生物氯化钾协同转运蛋白的调节胞质域的结构。
阳离子-氯化物协同转运蛋白 (CCC) 调节氯跨膜的运动,控制从细胞体积维持到神经元信号传导的生理过程。人类 CCC 是现有利尿剂和潜在的其他适应症的临床目标。在这里,我们报告了真核生物氯化钾协同转运蛋白Caenorhabditis elegans的可溶性 C 端调节域的 X 射线晶体结构KCC-1。我们观察到 CCC 中保守的核心 α/β 折叠。使用基于结构的序列比对,我们分析了与其他 CCC 家族成员 C 端域的异同。我们发现重要的调控基序位于结构较少的区域,对于二聚化重要的残基并未广泛保守,这表明寡聚化及其影响可能在更大的家族内有所不同。这张真核 KCC 的快照是合理设计细胞氯化物调节研究的一个有价值的起点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号