当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How does the thermal-aggregation behavior of black cricket protein isolate affect its foaming and gelling properties?
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.foodhyd.2020.106169 Livia A. Santiago , Orlando M. Fadel , Guilherme M. Tavares
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.foodhyd.2020.106169 Livia A. Santiago , Orlando M. Fadel , Guilherme M. Tavares
|
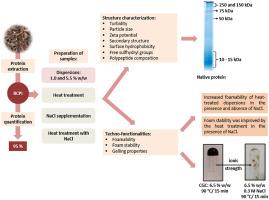
Abstract Edible insects are a promising source of high-quality proteins and their use to produce protein-rich ingredients for formulated foods has been pointed out as a good alternative to increase their acceptance by consumers. In order to understand the technological potential of these novel ingredients, it is crucial to characterize their behavior during food processing. In this context, this study aimed to evaluate the effect of heat treatments (65, 75, 85, 90 and 95 °C for 15 min) at variable ionic strength (from 0 up to 0.5 M of NaCl) on the structure, gelling and foaming properties of black cricket (Gryllus assimilis) protein isolate (BCPI). In absence of added NaCl, heat treatments do not considerably change the secondary structure of the proteins, although leaded to an increase of their surface hydrophobicity. On the other hand, heat treatment in the presence of NaCl induced a decrease in the content of the α-helix structure and the surface hydrophobicity of the proteins. The foamability of heat treated (at 75 and 95 °C for 15 min) dispersions supplemented or not with NaCl was similar to the one of whey protein isolate (WPI) dispersion. In addition, the BCPI dispersion heat treated at 90 °C for 15 min had a critical gelling concentration of 6.5% w/w, also comparable to WPI, which reveals its technological potential for food applications.
中文翻译:

黑蟋蟀分离蛋白的热聚集行为如何影响其发泡和胶凝特性?
摘要 食用昆虫是一种很有前途的优质蛋白质来源,它们用于生产用于配制食品的富含蛋白质的成分已被指出是提高消费者接受度的良好替代品。为了了解这些新成分的技术潜力,在食品加工过程中表征它们的行为至关重要。在此背景下,本研究旨在评估在不同离子强度(从 0 到 0.5 M 的 NaCl)下热处理(65、75、85、90 和 95 °C,持续 15 分钟)对结构、胶凝和黑蟋蟀 (Gryllus assimilis) 分离蛋白 (BCPI) 的发泡特性。在不添加 NaCl 的情况下,热处理不会显着改变蛋白质的二级结构,尽管会导致其表面疏水性增加。另一方面,在 NaCl 存在下的热处理导致 α-螺旋结构的含量和蛋白质的表面疏水性降低。添加或不添加 NaCl 的热处理(在 75 和 95 °C 下 15 分钟)分散体的发泡性类似于乳清蛋白分离物 (WPI) 分散体之一。此外,在 90°C 下热处理 15 分钟的 BCPI 分散体具有 6.5% w/w 的临界胶凝浓度,也与 WPI 相当,这显示了其在食品应用中的技术潜力。
更新日期:2021-01-01
中文翻译:

黑蟋蟀分离蛋白的热聚集行为如何影响其发泡和胶凝特性?
摘要 食用昆虫是一种很有前途的优质蛋白质来源,它们用于生产用于配制食品的富含蛋白质的成分已被指出是提高消费者接受度的良好替代品。为了了解这些新成分的技术潜力,在食品加工过程中表征它们的行为至关重要。在此背景下,本研究旨在评估在不同离子强度(从 0 到 0.5 M 的 NaCl)下热处理(65、75、85、90 和 95 °C,持续 15 分钟)对结构、胶凝和黑蟋蟀 (Gryllus assimilis) 分离蛋白 (BCPI) 的发泡特性。在不添加 NaCl 的情况下,热处理不会显着改变蛋白质的二级结构,尽管会导致其表面疏水性增加。另一方面,在 NaCl 存在下的热处理导致 α-螺旋结构的含量和蛋白质的表面疏水性降低。添加或不添加 NaCl 的热处理(在 75 和 95 °C 下 15 分钟)分散体的发泡性类似于乳清蛋白分离物 (WPI) 分散体之一。此外,在 90°C 下热处理 15 分钟的 BCPI 分散体具有 6.5% w/w 的临界胶凝浓度,也与 WPI 相当,这显示了其在食品应用中的技术潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号