当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Integrated In Silico Models for the Prediction of No-Observed-(Adverse)-Effect Levels and Lowest-Observed-(Adverse)-Effect Levels in Rats for Sub-chronic Repeated-Dose Toxicity
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-07-15 , DOI: 10.1021/acs.chemrestox.0c00176 Domenico Gadaleta 1 , Marco Marzo 1 , Andrey Toropov 1 , Alla Toropova 1 , Giovanna J Lavado 1 , Sylvia E Escher 2 , Jean Lou C M Dorne 3 , Emilio Benfenati 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-07-15 , DOI: 10.1021/acs.chemrestox.0c00176 Domenico Gadaleta 1 , Marco Marzo 1 , Andrey Toropov 1 , Alla Toropova 1 , Giovanna J Lavado 1 , Sylvia E Escher 2 , Jean Lou C M Dorne 3 , Emilio Benfenati 1
Affiliation
|
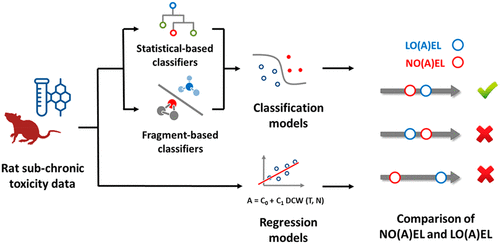
Repeated-dose toxicity (RDT) is a critical endpoint for hazard characterization of chemicals and is assessed to derive safe levels of exposure for human health. Here we present the first attempt to model simultaneously no-observed-(adverse)-effect level (NO(A)EL) and lowest-observed-(adverse)-effect level (LO(A)EL). Classification and regression models were derived based on rat sub-chronic repeated dose toxicity data for 327 compounds from the Fraunhofer RepDose database. Multi-category classification models were built for both NO(A)EL and LO(A)EL though a consensus of statistics- and fragment-based algorithms, while regression models were based on quantitative relationships between the endpoints and SMILES-based attributes. NO(A)EL and LO(A)EL models were integrated, and predictions were compared to exclude inconsistent values. This strategy improved the performance of single models, leading to R2 greater than 0.70, root-mean-square error (RMSE) lower than 0.60 (for regression models), and accuracy of 0.61–0.73 (for classification models) on the validation set, based on the endpoint and the threshold applied for selecting predictions. This study confirms the effectiveness of the modeling strategy presented here for assessing RDT of chemicals using in silico models.
中文翻译:

用于预测大鼠亚慢性重复剂量毒性的未观察到(不良)效应水平和最低观察到(不良)效应水平的集成计算机模型
重复剂量毒性 (RDT) 是化学品危害特征描述的关键终点,经过评估可得出对人类健康的安全暴露水平。在这里,我们首次尝试同时模拟未观察到(不良)效应水平(NO(A)EL)和最低观察到(不良)效应水平(LO(A)EL)。分类和回归模型是基于来自 Fraunhofer RepDose 数据库的 327 种化合物的大鼠亚慢性重复剂量毒性数据得出的。通过基于统计和片段的算法的共识,为 NO(A)EL 和 LO(A)EL 构建了多类别分类模型,而回归模型基于端点和基于 SMILES 的属性之间的定量关系。整合 NO(A)EL 和 LO(A)EL 模型,并比较预测以排除不一致的值。R 2大于 0.70,均方根误差 (RMSE) 小于 0.60(对于回归模型),验证集的准确度为 0.61–0.73(对于分类模型),基于终点和用于选择的阈值预测。这项研究证实了此处介绍的建模策略的有效性,用于评估使用计算机模型的化学品的 RDT 。
更新日期:2020-07-15
中文翻译:

用于预测大鼠亚慢性重复剂量毒性的未观察到(不良)效应水平和最低观察到(不良)效应水平的集成计算机模型
重复剂量毒性 (RDT) 是化学品危害特征描述的关键终点,经过评估可得出对人类健康的安全暴露水平。在这里,我们首次尝试同时模拟未观察到(不良)效应水平(NO(A)EL)和最低观察到(不良)效应水平(LO(A)EL)。分类和回归模型是基于来自 Fraunhofer RepDose 数据库的 327 种化合物的大鼠亚慢性重复剂量毒性数据得出的。通过基于统计和片段的算法的共识,为 NO(A)EL 和 LO(A)EL 构建了多类别分类模型,而回归模型基于端点和基于 SMILES 的属性之间的定量关系。整合 NO(A)EL 和 LO(A)EL 模型,并比较预测以排除不一致的值。R 2大于 0.70,均方根误差 (RMSE) 小于 0.60(对于回归模型),验证集的准确度为 0.61–0.73(对于分类模型),基于终点和用于选择的阈值预测。这项研究证实了此处介绍的建模策略的有效性,用于评估使用计算机模型的化学品的 RDT 。









































 京公网安备 11010802027423号
京公网安备 11010802027423号