当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sodium Drives Interfacial Equilibria for Semi-Soluble Phosphoric and Phosphonic Acids of Model Sea Spray Aerosol Surfaces
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-07-14 , DOI: 10.1021/acsearthspacechem.0c00132 Jennifer F. Neal 1 , Mickey M. Rogers 1 , Morgan A. Smeltzer 1 , Kimberly A. Carter-Fenk 1 , Alexander J. Grooms 1 , Mia M. Zerkle 1 , Heather C. Allen 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-07-14 , DOI: 10.1021/acsearthspacechem.0c00132 Jennifer F. Neal 1 , Mickey M. Rogers 1 , Morgan A. Smeltzer 1 , Kimberly A. Carter-Fenk 1 , Alexander J. Grooms 1 , Mia M. Zerkle 1 , Heather C. Allen 1
Affiliation
|
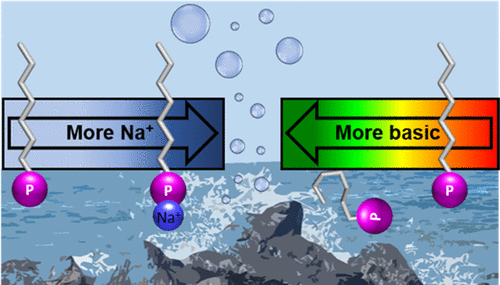
Organic phosphates and phosphonates represent important yet understudied constituents in our molecular understanding of the ocean. Herein, we determined the critical concentration of sodium relating to the onset of surface activity of alkyl phosphates and phosphonates at the air–water interface to further understand the interfacial environment of sea spray aerosols emitted from the ocean’s surface. A low pH range (1–5.6) was chosen to represent a model system for aged, acidic marine aerosols. The protonation state and sodium binding properties of C16–C18 alkyl phosphoric and phosphonic acids were explored using surface pressure–area isotherms and infrared reflection–absorption spectroscopy. We found that increasing pH and headgroup charge led to significant desorption of these semi-soluble phosphorus-containing acids into bulk solution, while the neutral, fully protonated, and sodium complexed species were favored at the interface. For the phosphonate species, the competition between sodium complexation and protonation reveals a critical sodium chloride concentration of ≥2 M at pH 2 necessary to outcompete the acid–base equilibrium. The onset of this equilibrium shift begins at concentrations as low as 0.1 M NaCl at pH 2, which demonstrates that ion pairing-mediated surface activity is highly relevant in sea spray aerosol systems. We also show that competitive interfacial equilibria between speciation and binding cannot be modeled by known bulk processes for the fully soluble methylphosphonic acid or through theoretical predictions from the Gouy–Chapman model.
中文翻译:

钠驱动模型海上喷雾气溶胶表面的半水溶性磷酸和膦酸的界面平衡
在我们对海洋的分子理解中,有机磷酸盐和膦酸盐是重要但尚未被研究的成分。在此,我们确定了与空气-水界面处的烷基磷酸盐和膦酸盐的表面活性的发生有关的钠的临界浓度,以进一步了解从海洋表面排放的海浪气溶胶的界面环境。选择低pH范围(1–5.6)代表老化的酸性海洋气溶胶的模型系统。C 16 –C 18的质子化状态和钠结合性质使用表面压力-面积等温线和红外反射-吸收光谱法研究了烷基磷酸和膦酸。我们发现,增加pH值和头基电荷会导致这些半可溶性含磷酸大量解吸到本体溶液中,而在界面处倾向于中性,完全质子化和钠络合的物种。对于膦酸酯类,钠络合和质子化之间的竞争表明,在2的临界氯化钠浓度下,必须要超过酸碱平衡才能达到≥2M。这种平衡转变的开始是在pH 2下低至0.1 M NaCl的浓度开始的,这表明离子配对介导的表面活性在海水喷雾气溶胶系统中高度相关。
更新日期:2020-09-18
中文翻译:

钠驱动模型海上喷雾气溶胶表面的半水溶性磷酸和膦酸的界面平衡
在我们对海洋的分子理解中,有机磷酸盐和膦酸盐是重要但尚未被研究的成分。在此,我们确定了与空气-水界面处的烷基磷酸盐和膦酸盐的表面活性的发生有关的钠的临界浓度,以进一步了解从海洋表面排放的海浪气溶胶的界面环境。选择低pH范围(1–5.6)代表老化的酸性海洋气溶胶的模型系统。C 16 –C 18的质子化状态和钠结合性质使用表面压力-面积等温线和红外反射-吸收光谱法研究了烷基磷酸和膦酸。我们发现,增加pH值和头基电荷会导致这些半可溶性含磷酸大量解吸到本体溶液中,而在界面处倾向于中性,完全质子化和钠络合的物种。对于膦酸酯类,钠络合和质子化之间的竞争表明,在2的临界氯化钠浓度下,必须要超过酸碱平衡才能达到≥2M。这种平衡转变的开始是在pH 2下低至0.1 M NaCl的浓度开始的,这表明离子配对介导的表面活性在海水喷雾气溶胶系统中高度相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号