当前位置:
X-MOL 学术
›
Batteries Supercaps
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analysis of Carbonate Decomposition During Solid Electrolyte Interphase Formation in Isotope‐Labeled Lithium Ion Battery Electrolytes: Extending the Knowledge about Electrolyte Soluble Species
Batteries & Supercaps ( IF 5.1 ) Pub Date : 2020-07-15 , DOI: 10.1002/batt.202000170 Christoph Peschel 1 , Fabian Horsthemke 1 , Marco Leißing 1 , Simon Wiemers‐Meyer 1 , Jonas Henschel 1 , Martin Winter 1, 2 , Sascha Nowak 1
Batteries & Supercaps ( IF 5.1 ) Pub Date : 2020-07-15 , DOI: 10.1002/batt.202000170 Christoph Peschel 1 , Fabian Horsthemke 1 , Marco Leißing 1 , Simon Wiemers‐Meyer 1 , Jonas Henschel 1 , Martin Winter 1, 2 , Sascha Nowak 1
Affiliation
|
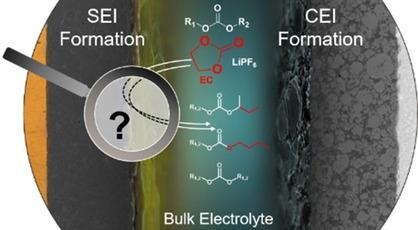
Sufficient interphase formation during the first cycles is crucial for the long‐term performance of lithium ion batteries. During the first cycles, electrolyte salt and solvent molecule decomposition caused by electrochemical instabilities leads to a wide range of decomposition species contributing to the formation of performance‐beneficial interphases at the electrodes as well as performance‐impairing side reactions. Due to structural similarities of carbonate educts, elucidation of underlying reaction pathways is complex. In this work, isotope‐labeled ethylene carbonate (13C3−EC) is applied to differentiate contributions of cyclic and linear carbonates to occurring interphase and decomposition reactions. Thereby, carbon atom tracing of electrolyte soluble species is performed by means of gas chromatography‐mass spectrometry (GC−MS). Reaction pathways are postulated based on identified molecular origins. Among others, a new DMC‐EC cross reactivity via lithium ethylene monocarbonate is considered. Moreover, radical reactions resulting in C−C bond formation are investigated and radical polymerizations of stoichiometric equivalents of ethene formed in situ during EC reduction are proposed. Finally, alkyl carbonates with C>4 alkyl chains are identified considering underlying reactivities. Discussed electrolyte decomposition reactions extend the understanding of EC‐based solid electrolyte interphase (SEI) formation emphasizing possible radical reactivities and solvent molecule contributions.
中文翻译:

同位素标记的锂离子电池电解质中固体电解质相间形成过程中碳酸盐分解的分析:扩展关于电解质可溶性物种的知识
在第一个循环中足够的相间形成对于锂离子电池的长期性能至关重要。在第一个循环中,由电化学不稳定性引起的电解质盐和溶剂分子分解会导致各种各样的分解物,这些分解物有助于在电极上形成对性能有益的中间相以及损害性能的副反应。由于碳酸盐离析物的结构相似性,阐明潜在的反应途径非常复杂。在这项工作中,同位素标记的碳酸亚乙酯(13 C 3-EC)用于区分环状碳酸酯和线性碳酸酯对发生的相间和分解反应的贡献。因此,通过气相色谱-质谱法(GC-MS)对电解质可溶物质的碳原子进行追踪。根据确定的分子来源推测反应途径。除其他外,还考虑了一种新的DMC-EC通过单碳酸锂锂的交叉反应性。此外,研究了导致CC键形成的自由基反应,并提出了在EC还原过程中原位形成的乙烯的化学计量当量的自由基聚合。最后,C > 4的碳酸烷基酯考虑到潜在的反应性确定烷基链。讨论过的电解质分解反应扩展了对基于EC的固体电解质相间(SEI)形成的理解,强调可能的自由基反应性和溶剂分子的贡献。
更新日期:2020-07-15
中文翻译:

同位素标记的锂离子电池电解质中固体电解质相间形成过程中碳酸盐分解的分析:扩展关于电解质可溶性物种的知识
在第一个循环中足够的相间形成对于锂离子电池的长期性能至关重要。在第一个循环中,由电化学不稳定性引起的电解质盐和溶剂分子分解会导致各种各样的分解物,这些分解物有助于在电极上形成对性能有益的中间相以及损害性能的副反应。由于碳酸盐离析物的结构相似性,阐明潜在的反应途径非常复杂。在这项工作中,同位素标记的碳酸亚乙酯(13 C 3-EC)用于区分环状碳酸酯和线性碳酸酯对发生的相间和分解反应的贡献。因此,通过气相色谱-质谱法(GC-MS)对电解质可溶物质的碳原子进行追踪。根据确定的分子来源推测反应途径。除其他外,还考虑了一种新的DMC-EC通过单碳酸锂锂的交叉反应性。此外,研究了导致CC键形成的自由基反应,并提出了在EC还原过程中原位形成的乙烯的化学计量当量的自由基聚合。最后,C > 4的碳酸烷基酯考虑到潜在的反应性确定烷基链。讨论过的电解质分解反应扩展了对基于EC的固体电解质相间(SEI)形成的理解,强调可能的自由基反应性和溶剂分子的贡献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号