当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aging aggravated liver ischemia and reperfusion injury by promoting STING-mediated NLRP3 activation in macrophages.
Aging Cell ( IF 8.0 ) Pub Date : 2020-07-14 , DOI: 10.1111/acel.13186 Weizhe Zhong 1, 2, 3 , Zhuqing Rao 4 , Jianhua Rao 1, 2, 3 , Guoyong Han 1, 2, 3 , Ping Wang 1, 2, 3 , Tao Jiang 1, 2, 3 , Xiongxiong Pan 4 , Shun Zhou 1, 2, 3 , Haoming Zhou 1, 2, 3 , Xuehao Wang 1, 2, 3
Aging Cell ( IF 8.0 ) Pub Date : 2020-07-14 , DOI: 10.1111/acel.13186 Weizhe Zhong 1, 2, 3 , Zhuqing Rao 4 , Jianhua Rao 1, 2, 3 , Guoyong Han 1, 2, 3 , Ping Wang 1, 2, 3 , Tao Jiang 1, 2, 3 , Xiongxiong Pan 4 , Shun Zhou 1, 2, 3 , Haoming Zhou 1, 2, 3 , Xuehao Wang 1, 2, 3
Affiliation
|
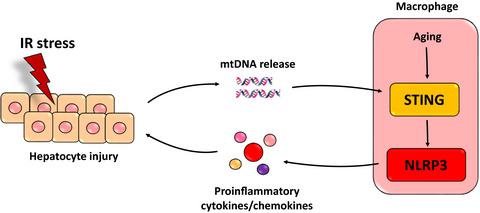
Although aggravated liver injury has been reported in aged livers post‐ischemia and reperfusion (IR), the underlying mechanism of innate immune activation of aged macrophages is not well understood. Here, we investigated whether and how Stimulator of interferon genes (STING) signaling regulated macrophage proinflammatory activation and liver IR injury. Mice were subjected to hepatic IR in vivo. Macrophages isolated from IR‐stressed livers and bone marrow‐derived macrophages (BMDMs) from young and aged mice were used for in vitro studies. Enhanced nucleotide‐binding domain and leucine‐rich repeat containing protein 3 (NLRP3) activation was found in both livers and macrophages of aged mice post‐IR. NLRP3 knockdown in macrophages inhibited intrahepatic inflammation and liver injury in both young and aged mice. Interestingly, enhanced activation of the STING/ TANK‐binding kinase 1 (TBK1) signaling pathway was observed in aged macrophages post‐IR and mitochondria DNA (mtDNA) stimulation. STING suppression blocked over‐activation of NLRP3 signaling and excessive secretion of proinflammatory cytokines/chemokines in the mtDNA‐stimulated BMDMs from aged mice. More importantly, STING knockdown in macrophages abrogated the detrimental role of aging in aggravating liver IR injury and intrahepatic inflammation. Finally, peripheral blood from the recipients undergoing liver transplantation was collected and analyzed. The results showed that the elderly recipients had much higher levels of TNF‐α, IL‐6, IL‐1β, and IL‐18 post‐transplantation, indicating increased NLRP3 activation in lR‐stressed livers of elderly recipients. In summary, our study demonstrated that the STING‐NLRP3 axis was critical for the proinflammatory response of aged macrophages and would be a novel therapeutic target to reduce IR injury in elderly patients.
中文翻译:

衰老通过促进巨噬细胞中 STING 介导的 NLRP3 激活加重肝脏缺血和再灌注损伤。
尽管在缺血和再灌注(IR)后老年肝脏中报道了加重的肝损伤,但老年巨噬细胞先天免疫激活的潜在机制尚不清楚。在这里,我们研究了干扰素基因刺激剂 (STING) 信号是否以及如何调节巨噬细胞促炎激活和肝脏 IR 损伤。小鼠在体内接受肝脏 IR。从 IR 应激肝脏中分离的巨噬细胞和来自年轻和老年小鼠的骨髓衍生巨噬细胞 (BMDM) 用于体外研究。在 IR 后老年小鼠的肝脏和巨噬细胞中都发现了增强的核苷酸结合域和富含亮氨酸重复序列的蛋白 3 (NLRP3) 激活。巨噬细胞中的 NLRP3 敲低抑制了年轻和老年小鼠的肝内炎症和肝损伤。有趣的是,在 IR 和线粒体 DNA (mtDNA) 刺激后的衰老巨噬细胞中观察到 STING/TANK 结合激酶 1 (TBK1) 信号通路的激活增强。STING 抑制阻止了来自老年小鼠的 mtDNA 刺激的 BMDM 中 NLRP3 信号传导的过度激活和促炎细胞因子/趋化因子的过度分泌。更重要的是,巨噬细胞中的 STING 敲低消除了衰老在加重肝 IR 损伤和肝内炎症中的不利作用。最后,收集并分析接受肝移植受者的外周血。结果表明,老年受者移植后 TNF-α、IL-6、IL-1β 和 IL-18 水平高得多,表明老年受者受 lR 应激的肝脏中 NLRP3 激活增加。总之,
更新日期:2020-07-14
中文翻译:

衰老通过促进巨噬细胞中 STING 介导的 NLRP3 激活加重肝脏缺血和再灌注损伤。
尽管在缺血和再灌注(IR)后老年肝脏中报道了加重的肝损伤,但老年巨噬细胞先天免疫激活的潜在机制尚不清楚。在这里,我们研究了干扰素基因刺激剂 (STING) 信号是否以及如何调节巨噬细胞促炎激活和肝脏 IR 损伤。小鼠在体内接受肝脏 IR。从 IR 应激肝脏中分离的巨噬细胞和来自年轻和老年小鼠的骨髓衍生巨噬细胞 (BMDM) 用于体外研究。在 IR 后老年小鼠的肝脏和巨噬细胞中都发现了增强的核苷酸结合域和富含亮氨酸重复序列的蛋白 3 (NLRP3) 激活。巨噬细胞中的 NLRP3 敲低抑制了年轻和老年小鼠的肝内炎症和肝损伤。有趣的是,在 IR 和线粒体 DNA (mtDNA) 刺激后的衰老巨噬细胞中观察到 STING/TANK 结合激酶 1 (TBK1) 信号通路的激活增强。STING 抑制阻止了来自老年小鼠的 mtDNA 刺激的 BMDM 中 NLRP3 信号传导的过度激活和促炎细胞因子/趋化因子的过度分泌。更重要的是,巨噬细胞中的 STING 敲低消除了衰老在加重肝 IR 损伤和肝内炎症中的不利作用。最后,收集并分析接受肝移植受者的外周血。结果表明,老年受者移植后 TNF-α、IL-6、IL-1β 和 IL-18 水平高得多,表明老年受者受 lR 应激的肝脏中 NLRP3 激活增加。总之,











































 京公网安备 11010802027423号
京公网安备 11010802027423号