当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Superoxide Anion Disproportionation Induced by Li+ and H+ : Pathways to 1 O2 Release in Li-O2 Batteries.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-07-15 , DOI: 10.1002/cphc.202000318 Adriano Pierini 1 , Sergio Brutti 1 , Enrico Bodo 1
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-07-15 , DOI: 10.1002/cphc.202000318 Adriano Pierini 1 , Sergio Brutti 1 , Enrico Bodo 1
Affiliation
|
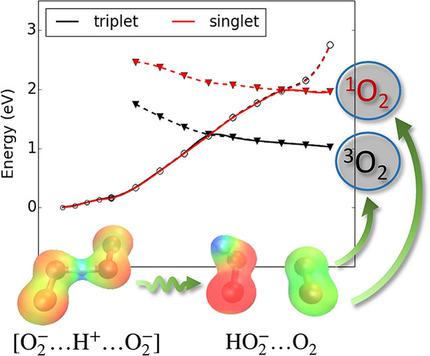
We explore the disproportionation reaction of superoxide anions in the presence of H+ and Li+ cations with high quality multiconfigurational ab‐initio methods. This reaction is of paramount importance in Li−O2 battery chemistry as it represents the source of a major degrading impurity, singlet molecular oxygen. For the first time, the thermodynamic and kinetic data of the reaction are drawn from an accurate theoretical model where the electronic structure of the reactant and products is treated at the necessary level of theory. Overall, the H+ catalyzed O2−+O2− disproportionation follows a very efficient thermodynamic and kinetic reaction path leading to neutral 3O2, 1O2 and peroxide anions. On the contrary, we have found that the Li+ catalysis promotes only the release of 3O2 whereas the 1O2 formation is energetically unfeasible at room temperature.
中文翻译:

Li +和H +引起的超氧阴离子歧化:Li-O2电池释放1 O2的途径。
我们使用高质量的多构象从头算方法探索了在H +和Li +阳离子存在下超氧阴离子的歧化反应。该反应在Li-O 2电池化学中至关重要,因为它代表了主要降解杂质单线态分子氧的来源。首次从精确的理论模型中获得了反应的热力学和动力学数据,其中以必要的理论水平处理了反应物和产物的电子结构。整体,将H +催化ö 2 - + O 2 -歧化如下的非常有效的热力学和动力学反应路径导致中性3 Ô 2,1 Ò 2和过氧化物阴离子。相反,我们发现Li +催化仅促进3 O 2的释放,而1 O 2的形成在室温下在能量上是不可行的。
更新日期:2020-09-15
中文翻译:

Li +和H +引起的超氧阴离子歧化:Li-O2电池释放1 O2的途径。
我们使用高质量的多构象从头算方法探索了在H +和Li +阳离子存在下超氧阴离子的歧化反应。该反应在Li-O 2电池化学中至关重要,因为它代表了主要降解杂质单线态分子氧的来源。首次从精确的理论模型中获得了反应的热力学和动力学数据,其中以必要的理论水平处理了反应物和产物的电子结构。整体,将H +催化ö 2 - + O 2 -歧化如下的非常有效的热力学和动力学反应路径导致中性3 Ô 2,1 Ò 2和过氧化物阴离子。相反,我们发现Li +催化仅促进3 O 2的释放,而1 O 2的形成在室温下在能量上是不可行的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号