当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water sorption equilibrium on 2-hydroxyethyl-trimethylammonium acetate in the temperature range 298.25–349.55K
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.fluid.2020.112758 Mauro Luberti , Christopher Olkis , Grady Bensted , Giulio Santori
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.fluid.2020.112758 Mauro Luberti , Christopher Olkis , Grady Bensted , Giulio Santori
|
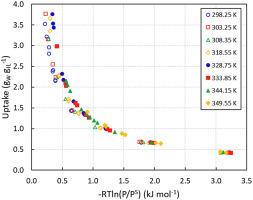
Abstract Ionic liquids (ILs) have emerged as novel sorption materials capable to achieve exceptional water vapour uptake that is attractive for their use in heat transformation technologies. Water sorption equilibria on 2-hydroxyethyl-trimethylammonium acetate or choline acetate ([Cho][OAc]) were measured at eight different temperatures: 298.25, 303.25, 308.35, 318.55, 328.75, 333.85, 344.15 and 349.55 K. Equilibrium measurements were performed with a dynamic vapour sorption (DVS) system. With a relative humidity of around 90% a water uptake larger than 3.5 gW gIL−1 was observed for all the temperatures. The resulting type III isotherms were correlated with the Guggenheim-Anderson-de Boer (GAB) model as well as the Redlich-Kister (RK) excess Gibbs energy model. It was found that both models fit the experimental data with great accuracy and provide an appropriate description of the water sorption equilibria. The regressed parameters of the RK model were also employed to predict excess properties of the binary system. Moreover, a thermodynamic cycle was assessed and compared with sorbent benchmarks, highlighting a performance that makes the choline acetate/water pair a viable option in sorption chiller applications.
中文翻译:

2-羟乙基-三甲基乙酸铵在 298.25–349.55K 温度范围内的吸水平衡
摘要 离子液体 (ILs) 已成为新型吸附材料,能够实现出色的水蒸气吸收,这对于它们在热转换技术中的应用很有吸引力。在八种不同温度下测量了 2-羟乙基-三甲基乙酸铵或乙酸胆碱 ([Cho][OAc]) 的水吸附平衡:298.25、303.25、308.35、318.55、328.75、333.85、344.95 和 344.95 和 344.95。动态蒸汽吸附 (DVS) 系统。在大约 90% 的相对湿度下,在所有温度下都观察到大于 3.5 gW gIL-1 的吸水量。得到的 III 型等温线与 Guggenheim-Anderson-de Boer (GAB) 模型以及 Redlich-Kister (RK) 过量 Gibbs 能量模型相关。发现这两种模型都非常准确地拟合了实验数据,并提供了对水吸附平衡的适当描述。RK 模型的回归参数也被用来预测二元系统的过度特性。此外,还评估了热力学循环并与吸附剂基准进行了比较,突出了使醋酸胆碱/水对成为吸附式制冷机应用中可行选择的性能。
更新日期:2020-11-01
中文翻译:

2-羟乙基-三甲基乙酸铵在 298.25–349.55K 温度范围内的吸水平衡
摘要 离子液体 (ILs) 已成为新型吸附材料,能够实现出色的水蒸气吸收,这对于它们在热转换技术中的应用很有吸引力。在八种不同温度下测量了 2-羟乙基-三甲基乙酸铵或乙酸胆碱 ([Cho][OAc]) 的水吸附平衡:298.25、303.25、308.35、318.55、328.75、333.85、344.95 和 344.95 和 344.95。动态蒸汽吸附 (DVS) 系统。在大约 90% 的相对湿度下,在所有温度下都观察到大于 3.5 gW gIL-1 的吸水量。得到的 III 型等温线与 Guggenheim-Anderson-de Boer (GAB) 模型以及 Redlich-Kister (RK) 过量 Gibbs 能量模型相关。发现这两种模型都非常准确地拟合了实验数据,并提供了对水吸附平衡的适当描述。RK 模型的回归参数也被用来预测二元系统的过度特性。此外,还评估了热力学循环并与吸附剂基准进行了比较,突出了使醋酸胆碱/水对成为吸附式制冷机应用中可行选择的性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号